

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM400802 QL-XII-51::US10000483, Compound I-11
SMILES: CC(C)(C)NS(=O)(=O)c1ccc(cc1)-c1ccc2ncc3ccc(=O)n(-c4ccc5CCN(C(=O)C=C)c5c4)c3c2c1
InChI Key: InChIKey=JXVBUPCEADJXCV-UHFFFAOYSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytoplasmic tyrosine-protein kinase BMX (Homo sapiens (Human)) | BDBM400802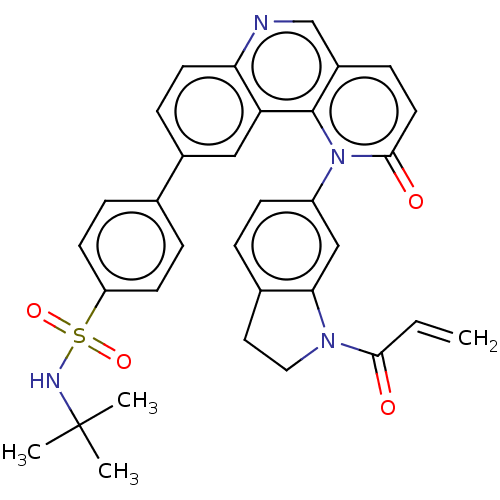 (QL-XII-51 | US10000483, Compound I-11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Purified BMX was mixed with substrate (FAK or Bmxtides), kinase buffer (final 20 mM HEPES, pH 7.5, 10 mM MgCl2, 20 mM β-glycerophosphate, 1 mM d... | J Med Chem 52: 6621-36 (2009) BindingDB Entry DOI: 10.7270/Q2QF8W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM400802 (QL-XII-51 | US10000483, Compound I-11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description DiscoverX binding assays were performed according to published methods (Fabian et al., Nat. Biotechnol. 23, 329-36 (2005); Davis et al., Nat. Biotech... | J Med Chem 52: 6621-36 (2009) BindingDB Entry DOI: 10.7270/Q2QF8W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic tyrosine-protein kinase BMX (Homo sapiens (Human)) | BDBM400802 (QL-XII-51 | US10000483, Compound I-11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description DiscoverX binding assays were performed according to published methods (Fabian et al., Nat. Biotechnol. 23, 329-36 (2005); Davis et al., Nat. Biotech... | J Med Chem 52: 6621-36 (2009) BindingDB Entry DOI: 10.7270/Q2QF8W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM400802 (QL-XII-51 | US10000483, Compound I-11) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China Curated by ChEMBL | Assay Description Inhibition of recombinant full length His-tagged human BLK expressed in baculovirus expression system using poly (4:1 Glu, Tyr) as substrate preincub... | Eur J Med Chem 137: 545-557 (2017) Article DOI: 10.1016/j.ejmech.2017.06.016 BindingDB Entry DOI: 10.7270/Q2NG4T51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM400802 (QL-XII-51 | US10000483, Compound I-11) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human JAK3 expressed in baculovirus expression system using poly (4:1 Glu, Tyr) as substrate preincubated for 1 ... | Eur J Med Chem 137: 545-557 (2017) Article DOI: 10.1016/j.ejmech.2017.06.016 BindingDB Entry DOI: 10.7270/Q2NG4T51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM400802 (QL-XII-51 | US10000483, Compound I-11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China Curated by ChEMBL | Assay Description Inhibition of BTK autophosphorylation at Y223 in anti-IgM stimulated human MOLM13 cells after 4 hrs by chemiluminescence assay | Eur J Med Chem 137: 545-557 (2017) Article DOI: 10.1016/j.ejmech.2017.06.016 BindingDB Entry DOI: 10.7270/Q2NG4T51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic tyrosine-protein kinase BMX (Homo sapiens (Human)) | BDBM400802 (QL-XII-51 | US10000483, Compound I-11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China Curated by ChEMBL | Assay Description Inhibition of recombinant full length His-tagged human BMX expressed in baculovirus expression system using poly (4:1 Glu, Tyr) as substrate preincub... | Eur J Med Chem 137: 545-557 (2017) Article DOI: 10.1016/j.ejmech.2017.06.016 BindingDB Entry DOI: 10.7270/Q2NG4T51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM400802 (QL-XII-51 | US10000483, Compound I-11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 536 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description DiscoverX binding assays were performed according to published methods (Fabian et al., Nat. Biotechnol. 23, 329-36 (2005); Davis et al., Nat. Biotech... | J Med Chem 52: 6621-36 (2009) BindingDB Entry DOI: 10.7270/Q2QF8W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||