

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM403653 (1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-N-[(1R,2R)-2-(difluoromethyl)-1-{[(1-methylcyclopropyl)sulfonyl]carbamoyl}cyclopropyl]-9-ethyl-18,18-difluoro-14-methoxy-1a-methyl-3,6-dioxo-1,1a,3,4,5,6,9,10,18,19,20,21,22,22a-tetradecahydro-8H-7,10-methanocyclopropa[18,19][1,10,3,6]dioxadiazacyclononadecino[11,12-b]quinoxaline-8-carboxamide::US10335409, Example 63
SMILES: CC[C@@H]1[C@H](CN([C@@H]1C(=O)OC(C)(C)C)C(=O)[C@@H](NC(=O)OC1(C)CC1CCC=C)C(C)(C)C)Oc1nc2cc(OC)ccc2nc1C(F)(F)C=C
InChI Key: InChIKey=HBVAPUQSLSYCOH-KJZHSGRXSA-N
Data: 2 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM403653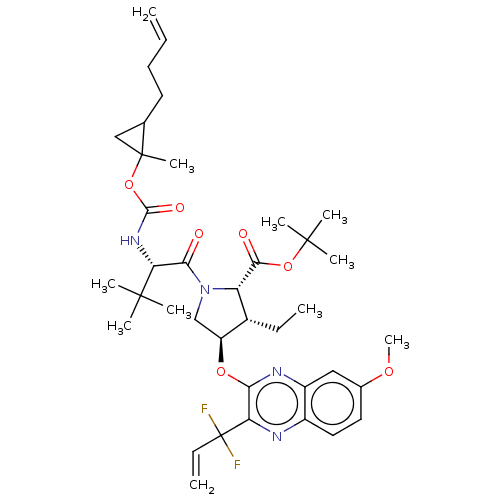 ((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-N-[(1R,2R)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NS3 serine protease (NS3) (Hepatitis C Virus (Virus)) | BDBM403653 ((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-N-[(1R,2R)-2-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||