

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM412611 N-(2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)phenyl)-6-methyl-8-(7-oxa-2-azaspiro[3.5]nonan-2-yl)pyrido[3,4-d]pyrimidin-2-amine::US10399974, Example 4
SMILES: CCOc1cc(ccc1Nc1ncc2cc(C)nc(N3CC4(C3)CCOCC4)c2n1)-c1nncn1C
InChI Key: InChIKey=JFSVDNNSGLCJII-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412611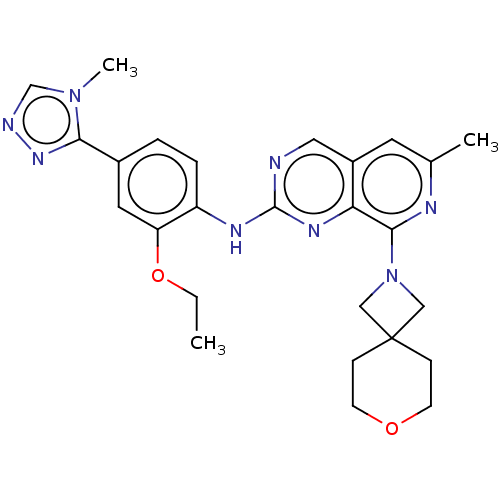 (N-(2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Dependent Kinase 2 (CDK2) (Homo sapiens (Human)) | BDBM412611 (N-(2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal His-tagged CDK2/cyclinA expressed in baculovirus expression system using 5FAM- peptide18 as substrate afte... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412611 (N-(2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description The enzyme reaction (total volume 10 μl) was carried out in black 384-well low volume plates containing full length MPS1 (12.5 nM or 3 nM), fluo... | US Patent US10399974 (2019) BindingDB Entry DOI: 10.7270/Q2K076NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412611 (N-(2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of MPS1 autophosphorylation at Thr33/Ser37 in human HCT116 cells after 2 hrs by MSD assay | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412611 (N-(2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Dependent Kinase 2 (CDK2) (Homo sapiens (Human)) | BDBM412611 (N-(2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal His-tagged CDK2/cyclinA expressed in baculovirus expression system using 5FAM- peptide18 as substrate afte... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||