

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM412675 US10399979, Compound 17b
SMILES: COc1cc2c3cnc4[nH]ccc4c3n(CC3CCCCC3)c(=O)c2cc1OC
InChI Key: InChIKey=RSFALMGMTQBFJZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM412675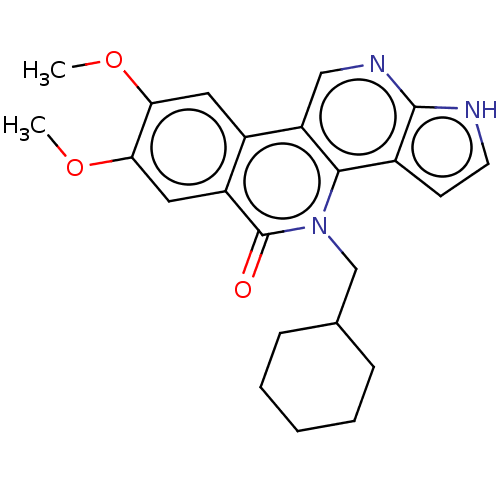 (US10399979, Compound 17b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Research Foundation US Patent | Assay Description Reagents used in JAK inhibition assay: Base Reaction buffer; 20 mM Hepes (pH 7.5), 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/ml BSA, 0.1 mM Na3VO... | US Patent US10399979 (2019) BindingDB Entry DOI: 10.7270/Q29G5Q52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Research Foundation US Patent | Assay Description Reagents used in JAK inhibition assay: Base Reaction buffer; 20 mM Hepes (pH 7.5), 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/ml BSA, 0.1 mM Na3VO... | US Patent US10399979 (2019) BindingDB Entry DOI: 10.7270/Q29G5Q52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Research Foundation US Patent | Assay Description Reagents used in JAK inhibition assay: Base Reaction buffer; 20 mM Hepes (pH 7.5), 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/ml BSA, 0.1 mM Na3VO... | US Patent US10399979 (2019) BindingDB Entry DOI: 10.7270/Q29G5Q52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 39.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Research Foundation US Patent | Assay Description Reagents used in JAK inhibition assay: Base Reaction buffer; 20 mM Hepes (pH 7.5), 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/ml BSA, 0.1 mM Na3VO... | US Patent US10399979 (2019) BindingDB Entry DOI: 10.7270/Q29G5Q52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NUAK family SNF1-like kinase 1 (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity to DNA-tagged recombinant human ARK5 (25 to 332 residues) expressed in bacterial expression system by KINOMEscan assay | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BMP-2-inducible protein kinase (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity to DNA-tagged recombinant human BIKE (34 to 329 residues) expressed in bacterial expression system by KINOMEscan assay | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity to DNA-tagged recombinant human FLT3 N841I mutant (592 to 969 residues) expressed in mammalian expression system by KINOMEscan assay | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity to DNA-tagged recombinant human TRKA (475 to 790 residues) expressed in mammalian expression system by KINOMEscan assay | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 7.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK3 in human SZ4 cells assessed as reduction in IL2-stimulated STAT5 phosphorylation preincubated for 1 hr followed by IL2 stimulation... | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NUAK family SNF1-like kinase 2 (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity to DNA-tagged recombinant human SNARK (22 to 333 residues) expressed in mammalian expression system by KINOMEscan assay | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||