

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM423464 WO2006061714, P38.7::WO2006061714-ID-09::cmdc.202100576, 10c
SMILES: CC(C)C[C@H](NC(=O)c1nc2ccccc2[nH]1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O
InChI Key: InChIKey=CQAHVFTUINWYCR-ZQIUZPCESA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM423464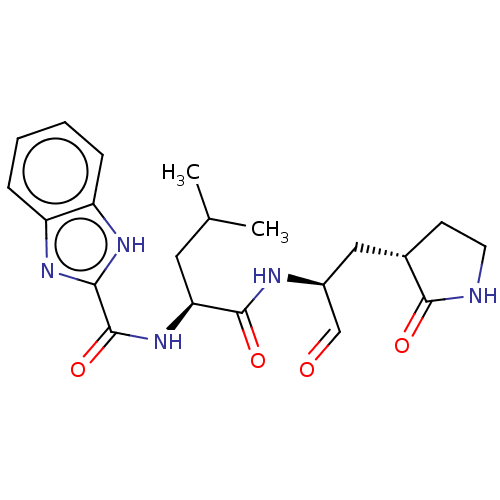 (WO2006061714, P38.7 | WO2006061714-ID-09 | cmdc.20...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description The SARS 3CLpro FRET assay measures the protease catalyzed cleavage of TAMRA- SITSAVLQSGFRKMK-(DABCYL)-OH to TAMRA - SITSAVLQ and SGFRKMK- (DABCYL)-O... | WIPO (2005) BindingDB Entry DOI: 10.7270/Q2PV6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM423464 (WO2006061714, P38.7 | WO2006061714-ID-09 | cmdc.20...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre | Assay Description Please point to the patents. | ChemMedChem (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3 like protease (SARS coronavirus Urbani) | BDBM423464 (WO2006061714, P38.7 | WO2006061714-ID-09 | cmdc.20...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO | n/a | n/a | 3.20E+4 | n/a | 3.60E+6 | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description Proteolytic activity of Coronavirus 3C protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS 3CI_pro FRET as... | WIPO (2006) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM423464 (WO2006061714, P38.7 | WO2006061714-ID-09 | cmdc.20...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO | n/a | n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description Protection from SARS Infection: Neutral Red Endpoint The ability of compounds to protect cells against infection by the SARS coronavirus is measured ... | WIPO (2005) BindingDB Entry DOI: 10.7270/Q2PV6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||