

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1cc(C(C)=O)c(Cl)cc1-n1nnc(c1C)S(=O)(=O)c1ccc(cc1)C(C)(C)C
InChI Key: InChIKey=IATNWTNALCFDJK-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429626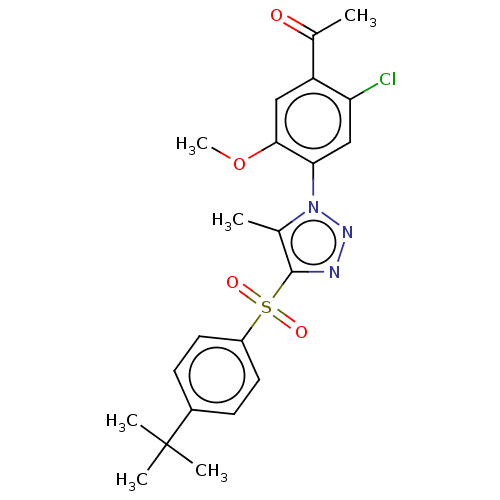 (US10550091, No. LC-35 | US10947203, No. LC-35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children''s Research Hospital US Patent | Assay Description The time-resolved fluorescence resonance transfer (TR-FRET) hPXR competitive binding assay was performed according to the manufacturer's instruct... | US Patent US10550091 (2020) BindingDB Entry DOI: 10.7270/Q2NK3HFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429626 (US10550091, No. LC-35 | US10947203, No. LC-35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
St. Jude Children''s Research Hospital US Patent | Assay Description The hPXR transactivation assays (PXR agonistic and antagonistic assays) were performed in the HepG2 cells stably expressing FLAG-hPXR and CYP3A4-luci... | US Patent US10550091 (2020) BindingDB Entry DOI: 10.7270/Q2NK3HFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429626 (US10550091, No. LC-35 | US10947203, No. LC-35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429626 (US10550091, No. LC-35 | US10947203, No. LC-35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
ST. JUDE CHILDREN''S RSEARCH HOSPITAL US Patent | Assay Description The hPXR transactivation assays (PXR agonistic and antagonistic assays) were performed in the HepG2 cells stably expressing FLAG-hPXR and CYP3A4-luci... | US Patent US10947203 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429626 (US10550091, No. LC-35 | US10947203, No. LC-35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ST. JUDE CHILDREN''S RSEARCH HOSPITAL US Patent | Assay Description The time-resolved fluorescence resonance transfer (TR-FRET) hPXR competitive binding assay was performed according to the manufacturer's instruct... | US Patent US10947203 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||