

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: NC1CCC(CC1)S(=O)(=O)N1[C@H]2CC[C@@H]1C[C@@H](C2)NC(=O)c1cc(on1)C1CC1
InChI Key: InChIKey=JYZQPJRVTADXNE-JSGMBBGDSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432822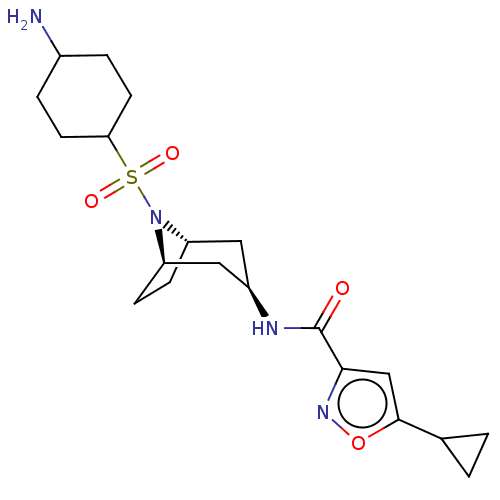 (N-((1R,3r,5S)-8-((4- aminocyclohexyl)sulfonyl)-8- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||