 Found 10 hits for monomerid = 433526
Found 10 hits for monomerid = 433526 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM433526
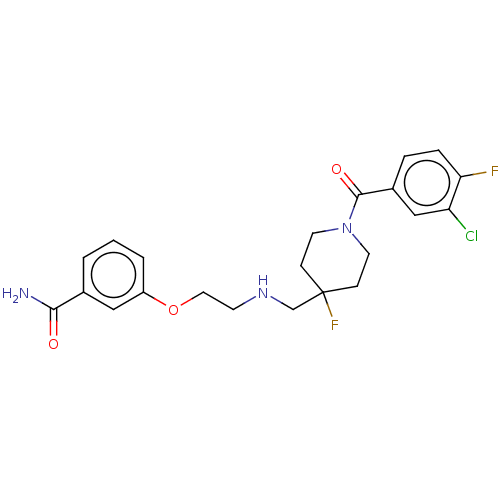
(US10562853, Compound 47)Show SMILES NC(=O)c1cccc(OCCNCC2(F)CCN(CC2)C(=O)c2ccc(F)c(Cl)c2)c1 Show InChI InChI=1S/C22H24ClF2N3O3/c23-18-13-16(4-5-19(18)24)21(30)28-9-6-22(25,7-10-28)14-27-8-11-31-17-3-1-2-15(12-17)20(26)29/h1-5,12-13,27H,6-11,14H2,(H2,26,29) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM433526

(US10562853, Compound 47)Show SMILES NC(=O)c1cccc(OCCNCC2(F)CCN(CC2)C(=O)c2ccc(F)c(Cl)c2)c1 Show InChI InChI=1S/C22H24ClF2N3O3/c23-18-13-16(4-5-19(18)24)21(30)28-9-6-22(25,7-10-28)14-27-8-11-31-17-3-1-2-15(12-17)20(26)29/h1-5,12-13,27H,6-11,14H2,(H2,26,29) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE
US Patent
| Assay Description
5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... |
US Patent US10562853 (2020)
BindingDB Entry DOI: 10.7270/Q29Z979D |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM433526

(US10562853, Compound 47)Show SMILES NC(=O)c1cccc(OCCNCC2(F)CCN(CC2)C(=O)c2ccc(F)c(Cl)c2)c1 Show InChI InChI=1S/C22H24ClF2N3O3/c23-18-13-16(4-5-19(18)24)21(30)28-9-6-22(25,7-10-28)14-27-8-11-31-17-3-1-2-15(12-17)20(26)29/h1-5,12-13,27H,6-11,14H2,(H2,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE
US Patent
| Assay Description
D2 Dopamine Receptor:Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human D2 receptor. All assays we... |
US Patent US10562853 (2020)
BindingDB Entry DOI: 10.7270/Q29Z979D |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM433526

(US10562853, Compound 47)Show SMILES NC(=O)c1cccc(OCCNCC2(F)CCN(CC2)C(=O)c2ccc(F)c(Cl)c2)c1 Show InChI InChI=1S/C22H24ClF2N3O3/c23-18-13-16(4-5-19(18)24)21(30)28-9-6-22(25,7-10-28)14-27-8-11-31-17-3-1-2-15(12-17)20(26)29/h1-5,12-13,27H,6-11,14H2,(H2,26,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE
US Patent
| Assay Description
Alpha1-adrenergic Receptor: Radioligand binding was performed using tissue (rat cortex). All assays were carried out in duplicates. 50 μL workin... |
US Patent US10562853 (2020)
BindingDB Entry DOI: 10.7270/Q29Z979D |
More data for this
Ligand-Target Pair | |
Alpha-1 Adrenergic Receptor/ adrenergic receptor/ adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM433526

(US10562853, Compound 47)Show SMILES NC(=O)c1cccc(OCCNCC2(F)CCN(CC2)C(=O)c2ccc(F)c(Cl)c2)c1 Show InChI InChI=1S/C22H24ClF2N3O3/c23-18-13-16(4-5-19(18)24)21(30)28-9-6-22(25,7-10-28)14-27-8-11-31-17-3-1-2-15(12-17)20(26)29/h1-5,12-13,27H,6-11,14H2,(H2,26,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM433526

(US10562853, Compound 47)Show SMILES NC(=O)c1cccc(OCCNCC2(F)CCN(CC2)C(=O)c2ccc(F)c(Cl)c2)c1 Show InChI InChI=1S/C22H24ClF2N3O3/c23-18-13-16(4-5-19(18)24)21(30)28-9-6-22(25,7-10-28)14-27-8-11-31-17-3-1-2-15(12-17)20(26)29/h1-5,12-13,27H,6-11,14H2,(H2,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM433526

(US10562853, Compound 47)Show SMILES NC(=O)c1cccc(OCCNCC2(F)CCN(CC2)C(=O)c2ccc(F)c(Cl)c2)c1 Show InChI InChI=1S/C22H24ClF2N3O3/c23-18-13-16(4-5-19(18)24)21(30)28-9-6-22(25,7-10-28)14-27-8-11-31-17-3-1-2-15(12-17)20(26)29/h1-5,12-13,27H,6-11,14H2,(H2,26,29) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 0.182 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM433526

(US10562853, Compound 47)Show SMILES NC(=O)c1cccc(OCCNCC2(F)CCN(CC2)C(=O)c2ccc(F)c(Cl)c2)c1 Show InChI InChI=1S/C22H24ClF2N3O3/c23-18-13-16(4-5-19(18)24)21(30)28-9-6-22(25,7-10-28)14-27-8-11-31-17-3-1-2-15(12-17)20(26)29/h1-5,12-13,27H,6-11,14H2,(H2,26,29) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 0.661 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM433526

(US10562853, Compound 47)Show SMILES NC(=O)c1cccc(OCCNCC2(F)CCN(CC2)C(=O)c2ccc(F)c(Cl)c2)c1 Show InChI InChI=1S/C22H24ClF2N3O3/c23-18-13-16(4-5-19(18)24)21(30)28-9-6-22(25,7-10-28)14-27-8-11-31-17-3-1-2-15(12-17)20(26)29/h1-5,12-13,27H,6-11,14H2,(H2,26,29) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM433526

(US10562853, Compound 47)Show SMILES NC(=O)c1cccc(OCCNCC2(F)CCN(CC2)C(=O)c2ccc(F)c(Cl)c2)c1 Show InChI InChI=1S/C22H24ClF2N3O3/c23-18-13-16(4-5-19(18)24)21(30)28-9-6-22(25,7-10-28)14-27-8-11-31-17-3-1-2-15(12-17)20(26)29/h1-5,12-13,27H,6-11,14H2,(H2,26,29) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data