

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM43868 3-[2-[4-[bis(4-fluorophenyl)methylene]piperidino]ethyl]-2-thioxo-1H-quinazolin-4-one::3-[2-[4-[bis(4-fluorophenyl)methylidene]-1-piperidinyl]ethyl]-2-sulfanylidene-1H-quinazolin-4-one::3-[2-[4-[bis(4-fluorophenyl)methylidene]piperidin-1-yl]ethyl]-2-sulfanylidene-1H-quinazolin-4-one::DIACYLGLYCEROL KINASE INHIBITOR II::MLS000069510::R 59949::SMR000058550::cid_657356
SMILES: Fc1ccc(cc1)C(=C1CCN(CCn2c(=S)[nH]c3ccccc3c2=O)CC1)c1ccc(F)cc1
InChI Key: InChIKey=ZCNBZFRECRPCKU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| electroneutral potassium-chloride cotransporter KCC2 (Homo sapiens (Human)) | BDBM43868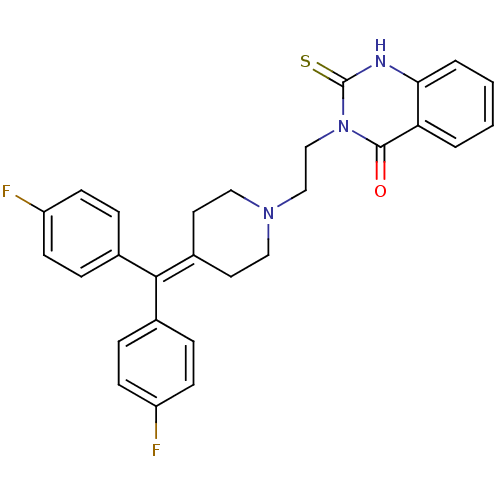 (3-[2-[4-[bis(4-fluorophenyl)methylene]piperidino]e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | PCBioAssay | n/a | n/a | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a |
Vanderbilt Screening Center for GPCRs, Ion Channels and Transporters Curated by PubChem BioAssay | Assay Description Vanderbilt Screening Center for GPCRs, Ion Channels and Transporters Assay Provider: Eric Delpire Assay Provider Affliation: Vanderbilt University Gr... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q23B5XKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| electroneutral potassium-chloride cotransporter KCC2 (Homo sapiens (Human)) | BDBM43868 (3-[2-[4-[bis(4-fluorophenyl)methylene]piperidino]e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | PCBioAssay | n/a | n/a | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a |
Vanderbilt Screening Center for GPCRs, Ion Channels and Transporters Curated by PubChem BioAssay | Assay Description Vanderbilt Screening Center for GPCRs, Ion Channels and Transporters Assay Provider: Eric Delpire Assay Provider Affliation: Vanderbilt University Gr... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2QV3JZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| electroneutral potassium-chloride cotransporter KCC2 (Homo sapiens (Human)) | BDBM43868 (3-[2-[4-[bis(4-fluorophenyl)methylene]piperidino]e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | PCBioAssay | n/a | n/a | n/a | n/a | 1.81E+5 | n/a | n/a | n/a | n/a |
Vanderbilt Screening Center for GPCRs, Ion Channels and Transporters Curated by PubChem BioAssay | Assay Description Vanderbilt Screening Center for GPCRs, Ion Channels and Transporters Assay Provider: Eric Delpire Assay Provider Affliation: Vanderbilt University Gr... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2M32T6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| electroneutral potassium-chloride cotransporter KCC2 (Homo sapiens (Human)) | BDBM43868 (3-[2-[4-[bis(4-fluorophenyl)methylene]piperidino]e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | PCBioAssay | n/a | n/a | n/a | n/a | 2.55E+4 | n/a | n/a | n/a | n/a |
Vanderbilt Screening Center for GPCRs, Ion Channels and Transporters Curated by PubChem BioAssay | Assay Description Vanderbilt Screening Center for GPCRs, Ion Channels and Transporters Assay Provider: Eric Delpire Assay Provider Affliation: Vanderbilt University Gr... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2PN942B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TPA: Essential protein of the mitochondrial intermembrane space (Saccharomyces cerevisiae S288c) | BDBM43868 (3-[2-[4-[bis(4-fluorophenyl)methylene]piperidino]e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | PCBioAssay | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2959G1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol kinase alpha (Homo sapiens) | BDBM43868 (3-[2-[4-[bis(4-fluorophenyl)methylene]piperidino]e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of OST-tagged DGKalpha (unknown origin) expressed in MDCK cell homogenates using DAG as substrate measured after 5 mins in presence of [ga... | Eur J Med Chem 164: 378-390 (2019) Article DOI: 10.1016/j.ejmech.2018.12.061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol kinase alpha (Homo sapiens) | BDBM43868 (3-[2-[4-[bis(4-fluorophenyl)methylene]piperidino]e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of OST-tagged DGKalpha (unknown origin) expressed in MDCK cell homogenates using DAG as substrate measured after 5 mins in presence of [ga... | Eur J Med Chem 164: 378-390 (2019) Article DOI: 10.1016/j.ejmech.2018.12.061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol kinase alpha (Sus scrofa) | BDBM43868 (3-[2-[4-[bis(4-fluorophenyl)methylene]piperidino]e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of pig FLAG3-tagged DGKalpha expressed in African green monkey COS7 cells using 1,2-dioleoyl-sn-glycerol as substrate measured after 30 mi... | Eur J Med Chem 164: 378-390 (2019) Article DOI: 10.1016/j.ejmech.2018.12.061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol kinase alpha (Homo sapiens) | BDBM43868 (3-[2-[4-[bis(4-fluorophenyl)methylene]piperidino]e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of DGKalpha in human erythrocytes using OAG as substrate preincubated for 1 min followed by OAG addition and measured after 8 mins in pres... | Eur J Med Chem 164: 378-390 (2019) Article DOI: 10.1016/j.ejmech.2018.12.061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol kinase alpha (Sus scrofa) | BDBM43868 (3-[2-[4-[bis(4-fluorophenyl)methylene]piperidino]e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of pig FLAG3-tagged DGKalpha expressed in African green monkey COS7 cells using 1,2-dioleoyl-sn-glycerol as substrate measured after 30 mi... | Eur J Med Chem 164: 378-390 (2019) Article DOI: 10.1016/j.ejmech.2018.12.061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol kinase alpha (Homo sapiens) | BDBM43868 (3-[2-[4-[bis(4-fluorophenyl)methylene]piperidino]e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of DGKalpha in human erythrocytes using OAG as substrate preincubated for 1 min followed by OAG addition and measured after 8 mins in pres... | Eur J Med Chem 164: 378-390 (2019) Article DOI: 10.1016/j.ejmech.2018.12.061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||