

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM444436 US10662159, Example 21
SMILES: COc1cc2CN(Cc2cc1OC)C(=O)OC(C(F)(F)F)C(F)(F)F
InChI Key: InChIKey=VZQAPCRSCJLLBW-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoacylglycerol lipase ABHD6 (Homo sapiens (Human)) | BDBM444436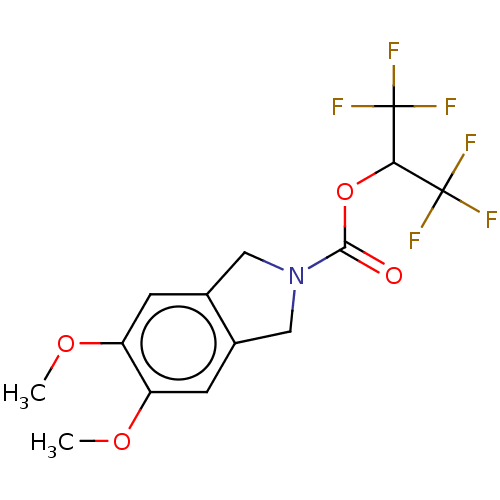 (US10662159, Example 21) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MAKSCIENTIFIC, LLC US Patent | Assay Description ABHD6: Certain compounds were tested for their ABHD6 and dual ABHD6/MGL inhibitory activity, which is expressed as % of inhibition or IC50 values. Th... | US Patent US10662159 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (aa 30-579) (Rattus norvegicus (rat)) | BDBM444436 (US10662159, Example 21) | PDB MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MAKSCIENTIFIC, LLC US Patent | Assay Description hABHD6:Initial Fluorescent Inhibition Assay (3-Point)—In each well of a 96-well plate 8 μL of membrane fraction containing full-length hABHD6 (1... | US Patent US10662159 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM444436 (US10662159, Example 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MAKSCIENTIFIC, LLC US Patent | Assay Description Rat/homo FAAH:Procedure was followed as described for hMGL, except that arachidonoyl-methyl coumarin (was used as fluorigenic substrate. Compounds we... | US Patent US10662159 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM444436 (US10662159, Example 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MAKSCIENTIFIC, LLC US Patent | Assay Description MGL: Compound inhibition of hMGL activity was assessed by a fluorometric assay recently developed in our laboratory (Makriyannis et al WO Patent Appl... | US Patent US10662159 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||