

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM46356 DIGITOXIN::MLS000069787::SMR000058529::US10668094, Compound Digitoxin::cid_441207
SMILES: C[C@H]1O[C@H](C[C@H](O)[C@@H]1O)O[C@H]1[C@@H](O)C[C@H](O[C@H]2[C@@H](O)C[C@H](O[C@H]3CC[C@@]4(C)[C@H](CC[C@@H]5[C@@H]4CC[C@]4(C)[C@H](CC[C@]54O)C4=CC(=O)OC4)C3)O[C@@H]2C)O[C@@H]1C
InChI Key: InChIKey=WDJUZGPOPHTGOT-XUDUSOBPSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na,K-ATPase alpha2beta3 (Homo sapiens (Human)) | BDBM46356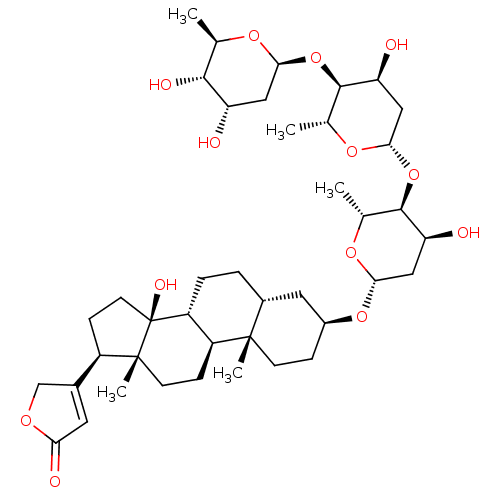 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 28.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yeda Research and Development Co. Ltd. US Patent | Assay Description To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo... | US Patent US10668094 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Na,K-ATPase alpha2beta1 (Homo sapiens (Human)) | BDBM46356 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 29.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yeda Research and Development Co. Ltd. US Patent | Assay Description To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo... | US Patent US10668094 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Na,K-ATPase alpha2beta2 (Homo sapiens (Human)) | BDBM46356 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 40.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yeda Research and Development Co. Ltd. US Patent | Assay Description To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo... | US Patent US10668094 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Na,K-ATPase alpha1beta1 (Homo sapiens (Human)) | BDBM46356 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yeda Research and Development Co. Ltd. US Patent | Assay Description To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo... | US Patent US10668094 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Canalicular multispecific organic anion transporter 1 (Homo sapiens (Human)) | BDBM46356 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 4 (Homo sapiens (Human)) | BDBM46356 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 4C1 (Homo sapiens) | BDBM46356 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Digoxin uptake in OATP4C1-expressing MDCK cells | Proc Natl Acad Sci U S A 101: 3569-74 (2004) Article DOI: 10.1073/pnas.0304987101 BindingDB Entry DOI: 10.7270/Q2WQ06NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM46356 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM46356 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea | Assay Description Ten-point DRCs were generated for each drug. Vero cells were seeded at 1.2 × 104 cells per well in DMEM, supplemented with 2% FBS and 1× ... | Antimicrob Agents Chemother 64: (2020) Article DOI: 10.1128/AAC.00819-20 BindingDB Entry DOI: 10.7270/Q22N54QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM46356 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-... | Drug Metab Dispos 40: 2332-41 (2012) Article DOI: 10.1124/dmd.112.047068 BindingDB Entry DOI: 10.7270/Q2ZP488M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/potassium-transporting ATPase alpha-1 chain (Canis familiaris) | BDBM46356 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manitoba Curated by ChEMBL | Assay Description Inhibition of [3H]ouabain binding to Digitalis receptor in dog heart microsomes | J Med Chem 40: 1439-46 (1997) Article DOI: 10.1021/jm960880l BindingDB Entry DOI: 10.7270/Q2BK1D1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transcription Factor STAT1 (Homo sapiens (Human)) | BDBM46356 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | >5.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2K935ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| STAT3 (Homo sapiens (Human)) | BDBM46356 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2TQ5ZXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Canalicular multispecific organic anion transporter 2 (Homo sapiens (Human)) | BDBM46356 (DIGITOXIN | MLS000069787 | SMR000058529 | US106680...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||