

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM466252 US10793582, Example 66
SMILES: O=C(NCc1ccccc1)[C@H]1CCCN1c1nc2ncnc(Oc3ccccc3)c2s1
InChI Key: InChIKey=HFFNKJUGEQTUEB-GOSISDBHSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466252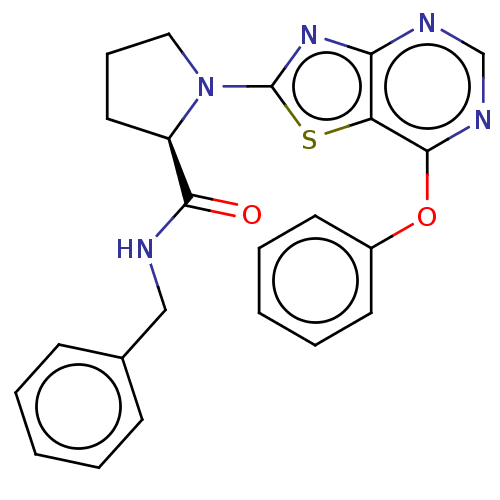 (US10793582, Example 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||