

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM482160 BDBM50396041::HG-10-102-01
SMILES: CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCOCC2)ncc1Cl
InChI Key: InChIKey=YEVOZZZLKJKCCD-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM482160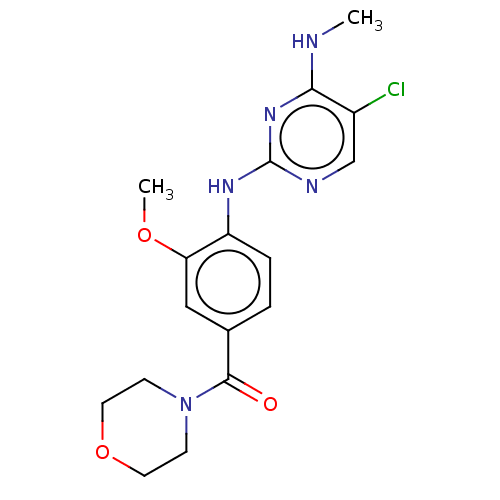 (BDBM50396041 | HG-10-102-01) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of LRRK2 | J Med Chem 55: 5536-45 (2012) Article DOI: 10.1021/jm300452p BindingDB Entry DOI: 10.7270/Q2RR20CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM482160 (BDBM50396041 | HG-10-102-01) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay | J Med Chem 55: 9416-33 (2012) Article DOI: 10.1021/jm301020q BindingDB Entry DOI: 10.7270/Q2P55PN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM482160 (BDBM50396041 | HG-10-102-01) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of JAK2 | J Med Chem 55: 5536-45 (2012) Article DOI: 10.1021/jm300452p BindingDB Entry DOI: 10.7270/Q2RR20CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GST-LRRK2 (aa 1326-2527,A2016T,G2019S) (Homo sapiens (Human)) | BDBM482160 (BDBM50396041 | HG-10-102-01) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 95.9 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC. US Patent | Assay Description Active GST-LRRK2 (1326-2527), GST-LRRK2[G2019S] (1326-2527), GST-LRRK2[A2016T] (1326-2527) and GST-LRRK2[A2016T+G2019S] (1326-2527) enzyme was purifi... | US Patent US10913744 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM482160 (BDBM50396041 | HG-10-102-01) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles River Curated by ChEMBL | Assay Description Inhibition of full length wild-type LRRK2 (unknown origin) using biotinylated ezrin/radaxin/meosin peptide as substrate measured after 1 hr | Bioorg Med Chem Lett 27: 2520-2527 (2017) Article DOI: 10.1016/j.bmcl.2017.03.098 BindingDB Entry DOI: 10.7270/Q2474D0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GST-LRRK2 (aa 1326-2527) (Homo sapiens (Human)) | BDBM482160 (BDBM50396041 | HG-10-102-01) | PDB GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 20.3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC. US Patent | Assay Description Active GST-LRRK2 (1326-2527), GST-LRRK2[G2019S] (1326-2527), GST-LRRK2[A2016T] (1326-2527) and GST-LRRK2[A2016T+G2019S] (1326-2527) enzyme was purifi... | US Patent US10913744 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GST-LRRK2 (aa 1326-2527,G2019S) (Homo sapiens (Human)) | BDBM482160 (BDBM50396041 | HG-10-102-01) | PDB GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC. US Patent | Assay Description Active GST-LRRK2 (1326-2527), GST-LRRK2[G2019S] (1326-2527), GST-LRRK2[A2016T] (1326-2527) and GST-LRRK2[A2016T+G2019S] (1326-2527) enzyme was purifi... | US Patent US10913744 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GST-LRRK2 (aa 1326-2527,A2016T) (Homo sapiens (Human)) | BDBM482160 (BDBM50396041 | HG-10-102-01) | PDB MMDB GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC. US Patent | Assay Description Active GST-LRRK2 (1326-2527), GST-LRRK2[G2019S] (1326-2527), GST-LRRK2[A2016T] (1326-2527) and GST-LRRK2[A2016T+G2019S] (1326-2527) enzyme was purifi... | US Patent US10913744 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM482160 (BDBM50396041 | HG-10-102-01) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LRRK2 expressed in HEK293 cells using nictide as substrate and [gamma32P]ATP after 15 mins by Cerenkov counting method | ACS Med Chem Lett 3: 658-662 (2012) Article DOI: 10.1021/ml300123a BindingDB Entry DOI: 10.7270/Q2FF3TF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 9 (MLK1) (Homo sapiens (Human)) | BDBM482160 (BDBM50396041 | HG-10-102-01) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of MLK1 by radiometric assay | ACS Med Chem Lett 3: 658-662 (2012) Article DOI: 10.1021/ml300123a BindingDB Entry DOI: 10.7270/Q2FF3TF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM482160 (BDBM50396041 | HG-10-102-01) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of MNK2 by radiometric assay | ACS Med Chem Lett 3: 658-662 (2012) Article DOI: 10.1021/ml300123a BindingDB Entry DOI: 10.7270/Q2FF3TF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM482160 (BDBM50396041 | HG-10-102-01) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of LRRK2 in human HEK293 cells | J Med Chem 55: 9416-33 (2012) Article DOI: 10.1021/jm301020q BindingDB Entry DOI: 10.7270/Q2P55PN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||