

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CN(C)C(=O)COc1ccc(cc1C=O)-c1cccc2[nH]ncc12
InChI Key: InChIKey=IXTRHVKNNCAPDT-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (2019-nCoV) | BDBM484197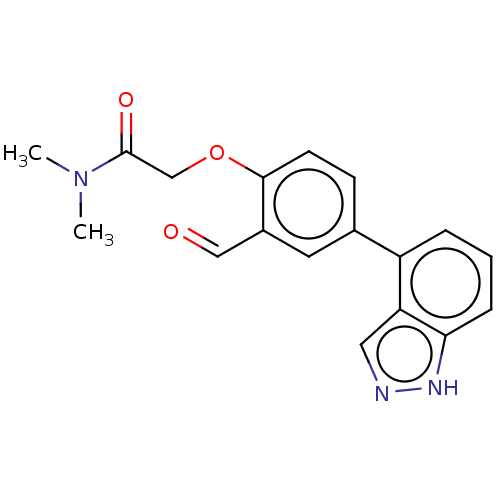 (CDD-1976 | WO2023004291, Compound CDD-1976) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine | Assay Description To determine the Ki values of active compounds, 25 nM Mpro was mixed with increasing concentrations of compounds (from 4 nM to 4,000 nM with twofold ... | Proc Natl Acad Sci U S A 118: (2021) Article DOI: 10.1073/pnas.2111172118 BindingDB Entry DOI: 10.7270/Q2W380FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM484197 (CDD-1976 | WO2023004291, Compound CDD-1976) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023004291 | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab-His6 (2019-nCoV) | BDBM484197 (CDD-1976 | WO2023004291, Compound CDD-1976) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023004291 | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM484197 (CDD-1976 | WO2023004291, Compound CDD-1976) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine | Assay Description Inhibition of SARS-CoV-2 replication in VERO E6 and HEK hACE2 cells was measured using an xCELLigence RTCA HT Analyzer (Agilent Technologies), tracki... | Proc Natl Acad Sci U S A 118: (2021) Article DOI: 10.1073/pnas.2111172118 BindingDB Entry DOI: 10.7270/Q2W380FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||