

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: C[C@H]1[C@H](O)CN1c1nc(c(C)c(n1)C12CNCC1C2CC(O)=O)C(F)(F)F
InChI Key: InChIKey=FIDCOOGXAYYGDL-WRCLSMMLSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ketohexokinase (Homo sapiens (Human)) | BDBM494880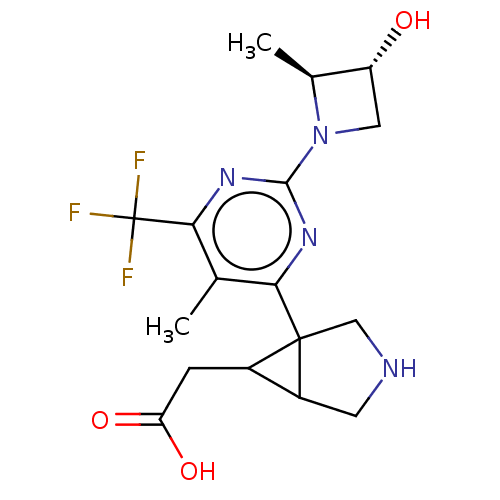 (US10988463, Example 24 | [(1R,5S,6R)-3-{2-[(2S,3R)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay A, a 384-well format on a Corning 3653 assay plate is used, and monitored by UV-vis spectroscopy in continuous mode at rt. Compounds were prepa... | US Patent US10988463 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM494880 (US10988463, Example 24 | [(1R,5S,6R)-3-{2-[(2S,3R)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay B, using 10-fold less enzyme and measuring absorbance for 3 hours to obtain IC50 values below the 10 nM lower limit of Assay A. Compounds were ... | US Patent US10988463 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM494880 (US10988463, Example 24 | [(1R,5S,6R)-3-{2-[(2S,3R)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay A, a 384-well format on a Corning 3653 assay plate is used, and monitored by UV-vis spectroscopy in continuous mode at rt. Compounds were prepa... | US Patent US10988463 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform A of Ketohexokinase (Peripheral) (Homo sapiens (Human)) | BDBM494880 (US10988463, Example 24 | [(1R,5S,6R)-3-{2-[(2S,3R)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay D, was performed using human KHK-A to assess the potency of compounds in inhibiting activity of this enzyme. Compounds were prepared in DMSO as... | US Patent US10988463 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM494880 (US10988463, Example 24 | [(1R,5S,6R)-3-{2-[(2S,3R)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay C, was performed at high fructose and ATP concentrations, conditions that would be more consistent with physiological concentrations of the nat... | US Patent US10988463 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||