 Found 10 hits for monomerid = 50001747
Found 10 hits for monomerid = 50001747 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet activating factor receptor
(Homo sapiens (Human)) | BDBM50001747
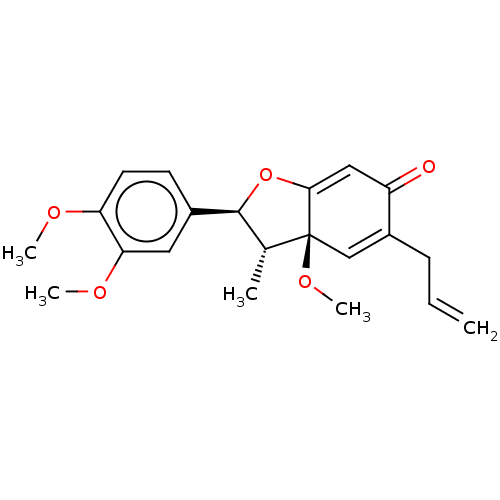
((+)-Kadsurenone | (Kadsurenone)5-Allyl-2-(3,4-dime...)Show SMILES COc1ccc(cc1OC)[C@H]1OC2=CC(=O)C(CC=C)=C[C@]2(OC)[C@@H]1C |c:20,t:13| Show InChI InChI=1S/C21H24O5/c1-6-7-15-12-21(25-5)13(2)20(26-19(21)11-16(15)22)14-8-9-17(23-3)18(10-14)24-4/h6,8-13,20H,1,7H2,2-5H3/t13-,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Platelet activating factor receptor
(Homo sapiens (Human)) | BDBM50001747

((+)-Kadsurenone | (Kadsurenone)5-Allyl-2-(3,4-dime...)Show SMILES COc1ccc(cc1OC)[C@H]1OC2=CC(=O)C(CC=C)=C[C@]2(OC)[C@@H]1C |c:20,t:13| Show InChI InChI=1S/C21H24O5/c1-6-7-15-12-21(25-5)13(2)20(26-19(21)11-16(15)22)14-8-9-17(23-3)18(10-14)24-4/h6,8-13,20H,1,7H2,2-5H3/t13-,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase (Cyclooxygenase-2)
(Ovis aries (Sheep)) | BDBM50001747

((+)-Kadsurenone | (Kadsurenone)5-Allyl-2-(3,4-dime...)Show SMILES COc1ccc(cc1OC)[C@H]1OC2=CC(=O)C(CC=C)=C[C@]2(OC)[C@@H]1C |c:20,t:13| Show InChI InChI=1S/C21H24O5/c1-6-7-15-12-21(25-5)13(2)20(26-19(21)11-16(15)22)14-8-9-17(23-3)18(10-14)24-4/h6,8-13,20H,1,7H2,2-5H3/t13-,20+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de Colombia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-2 assessed as inhibition of transformation of AA to PGH2 by EIA |
Bioorg Med Chem Lett 19: 6922-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.069
BindingDB Entry DOI: 10.7270/Q2J1043X |
More data for this
Ligand-Target Pair | |
Platelet activating factor receptor
(Cavia porcellus) | BDBM50001747

((+)-Kadsurenone | (Kadsurenone)5-Allyl-2-(3,4-dime...)Show SMILES COc1ccc(cc1OC)[C@H]1OC2=CC(=O)C(CC=C)=C[C@]2(OC)[C@@H]1C |c:20,t:13| Show InChI InChI=1S/C21H24O5/c1-6-7-15-12-21(25-5)13(2)20(26-19(21)11-16(15)22)14-8-9-17(23-3)18(10-14)24-4/h6,8-13,20H,1,7H2,2-5H3/t13-,20+,21+/m1/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitor concentration required to block 50% of the specific [3H]PAF binding to rabbit platelet membranes. |
J Med Chem 30: 136-42 (1987)
BindingDB Entry DOI: 10.7270/Q25T3M2X |
More data for this
Ligand-Target Pair | |
Platelet activating factor receptor
(Cavia porcellus) | BDBM50001747

((+)-Kadsurenone | (Kadsurenone)5-Allyl-2-(3,4-dime...)Show SMILES COc1ccc(cc1OC)[C@H]1OC2=CC(=O)C(CC=C)=C[C@]2(OC)[C@@H]1C |c:20,t:13| Show InChI InChI=1S/C21H24O5/c1-6-7-15-12-21(25-5)13(2)20(26-19(21)11-16(15)22)14-8-9-17(23-3)18(10-14)24-4/h6,8-13,20H,1,7H2,2-5H3/t13-,20+,21+/m1/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitor concentration required to block 50 %of the specific [3H]PAF binding to rabbit platelet membranes. |
J Med Chem 30: 136-42 (1987)
BindingDB Entry DOI: 10.7270/Q25T3M2X |
More data for this
Ligand-Target Pair | |
Platelet activating factor receptor
(Cavia porcellus) | BDBM50001747

((+)-Kadsurenone | (Kadsurenone)5-Allyl-2-(3,4-dime...)Show SMILES COc1ccc(cc1OC)[C@H]1OC2=CC(=O)C(CC=C)=C[C@]2(OC)[C@@H]1C |c:20,t:13| Show InChI InChI=1S/C21H24O5/c1-6-7-15-12-21(25-5)13(2)20(26-19(21)11-16(15)22)14-8-9-17(23-3)18(10-14)24-4/h6,8-13,20H,1,7H2,2-5H3/t13-,20+,21+/m1/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitor concentration required to block 50% of the specific [3H]PAF binding, obtained as natural product. |
J Med Chem 30: 136-42 (1987)
BindingDB Entry DOI: 10.7270/Q25T3M2X |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase (cyclooxygenase)
(Ovis aries (Sheep)) | BDBM50001747

((+)-Kadsurenone | (Kadsurenone)5-Allyl-2-(3,4-dime...)Show SMILES COc1ccc(cc1OC)[C@H]1OC2=CC(=O)C(CC=C)=C[C@]2(OC)[C@@H]1C |c:20,t:13| Show InChI InChI=1S/C21H24O5/c1-6-7-15-12-21(25-5)13(2)20(26-19(21)11-16(15)22)14-8-9-17(23-3)18(10-14)24-4/h6,8-13,20H,1,7H2,2-5H3/t13-,20+,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de Colombia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as inhibition of transformation of AA to PGH2 by EIA |
Bioorg Med Chem Lett 19: 6922-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.069
BindingDB Entry DOI: 10.7270/Q2J1043X |
More data for this
Ligand-Target Pair | |
Platelet activating factor receptor
(Cavia porcellus) | BDBM50001747

((+)-Kadsurenone | (Kadsurenone)5-Allyl-2-(3,4-dime...)Show SMILES COc1ccc(cc1OC)[C@H]1OC2=CC(=O)C(CC=C)=C[C@]2(OC)[C@@H]1C |c:20,t:13| Show InChI InChI=1S/C21H24O5/c1-6-7-15-12-21(25-5)13(2)20(26-19(21)11-16(15)22)14-8-9-17(23-3)18(10-14)24-4/h6,8-13,20H,1,7H2,2-5H3/t13-,20+,21+/m1/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition concentration required to block 50% of the specific [3H]PAF binding, obtained from Chiralpak column at low temperatures. |
J Med Chem 30: 136-42 (1987)
BindingDB Entry DOI: 10.7270/Q25T3M2X |
More data for this
Ligand-Target Pair | |
Platelet activating factor receptor
(Cavia porcellus) | BDBM50001747

((+)-Kadsurenone | (Kadsurenone)5-Allyl-2-(3,4-dime...)Show SMILES COc1ccc(cc1OC)[C@H]1OC2=CC(=O)C(CC=C)=C[C@]2(OC)[C@@H]1C |c:20,t:13| Show InChI InChI=1S/C21H24O5/c1-6-7-15-12-21(25-5)13(2)20(26-19(21)11-16(15)22)14-8-9-17(23-3)18(10-14)24-4/h6,8-13,20H,1,7H2,2-5H3/t13-,20+,21+/m1/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration required to inhibit PAF binding to rabbit platelet membrane |
Bioorg Med Chem Lett 1: 327-332 (1991)
Article DOI: 10.1016/S0960-894X(01)80818-0
BindingDB Entry DOI: 10.7270/Q2TM7BKP |
More data for this
Ligand-Target Pair | |
Platelet activating factor receptor
(Cavia porcellus) | BDBM50001747

((+)-Kadsurenone | (Kadsurenone)5-Allyl-2-(3,4-dime...)Show SMILES COc1ccc(cc1OC)[C@H]1OC2=CC(=O)C(CC=C)=C[C@]2(OC)[C@@H]1C |c:20,t:13| Show InChI InChI=1S/C21H24O5/c1-6-7-15-12-21(25-5)13(2)20(26-19(21)11-16(15)22)14-8-9-17(23-3)18(10-14)24-4/h6,8-13,20H,1,7H2,2-5H3/t13-,20+,21+/m1/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company
Curated by ChEMBL
| Assay Description
Inhibition of PAF-induced platelet aggregation in rabbit platelet rich plasma |
J Med Chem 36: 580-90 (1993)
BindingDB Entry DOI: 10.7270/Q2SX6C9X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data