

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50003407 CHEMBL3234568
SMILES: CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N
InChI Key: InChIKey=YMFDSPVIOOOQTH-HXUWFJFHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G-protein coupled bile acid receptor 1 (Mus musculus) | BDBM50003407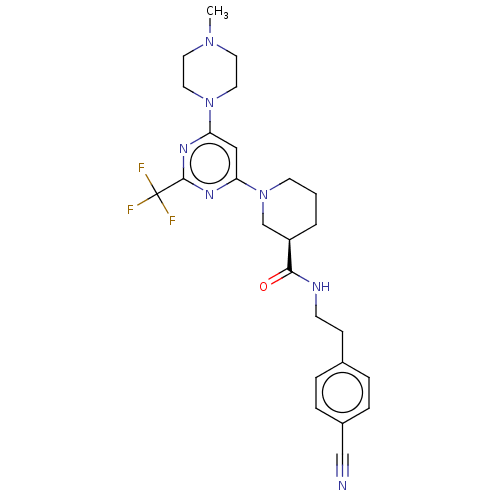 (CHEMBL3234568) | PDB Reactome pathway KEGG GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Agonist activity at mouse TGR5 expressed in Jurkat cells assessed as intracellular cAMP level after 1 hr by HTRF assay | J Med Chem 57: 3263-82 (2014) Article DOI: 10.1021/jm401731q BindingDB Entry DOI: 10.7270/Q2H41SZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50003407 (CHEMBL3234568) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 57: 3263-82 (2014) Article DOI: 10.1021/jm401731q BindingDB Entry DOI: 10.7270/Q2H41SZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional regulator ERG (Homo sapiens (Human)) | BDBM50003407 (CHEMBL3234568) | PDB MMDB KEGG B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human ERG by patch clamp method | J Med Chem 57: 3263-82 (2014) Article DOI: 10.1021/jm401731q BindingDB Entry DOI: 10.7270/Q2H41SZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled bile acid receptor 1 (Homo sapiens (Human)) | BDBM50003407 (CHEMBL3234568) | PDB Reactome pathway KEGG GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Agonist activity at human TGR5 expressed in Jurkat cells assessed as intracellular cAMP level after 1 hr by HTRF assay | J Med Chem 57: 3263-82 (2014) Article DOI: 10.1021/jm401731q BindingDB Entry DOI: 10.7270/Q2H41SZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled bile acid receptor 1 (Homo sapiens (Human)) | BDBM50003407 (CHEMBL3234568) | PDB Reactome pathway KEGG GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Agonist activity at TGR5 in human PBMC assessed as inhibition of LPS-induced TNFalpha production preincubated for 30 mins followed by LPS stimulation... | J Med Chem 57: 3263-82 (2014) Article DOI: 10.1021/jm401731q BindingDB Entry DOI: 10.7270/Q2H41SZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50003407 (CHEMBL3234568) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 57: 3263-82 (2014) Article DOI: 10.1021/jm401731q BindingDB Entry DOI: 10.7270/Q2H41SZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50003407 (CHEMBL3234568) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 57: 3263-82 (2014) Article DOI: 10.1021/jm401731q BindingDB Entry DOI: 10.7270/Q2H41SZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled bile acid receptor 1 (Mus musculus) | BDBM50003407 (CHEMBL3234568) | PDB Reactome pathway KEGG GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 296 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Agonist activity at TGR5 in mouse GLUTag cells assessed as secretion of GLP-1 after 2 hrs by HEK293 cell-based luciferase reporter gene assay in pres... | J Med Chem 57: 3263-82 (2014) Article DOI: 10.1021/jm401731q BindingDB Entry DOI: 10.7270/Q2H41SZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||