

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50006412 (3R,5R)-3,5-dihydroxy-7-((1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-((S)-2-methylbutanoyloxy)-1,2,6,7,8,8a-hexahydronaphthalen-1-yl)heptanoate sodium::3,5-dihydroxy-7-[8-hydroxy-3-methyl-10-[1-methyl-(1S)-propylcarbonyloxy]-(1R,2S,3S,8S,10S)-bicyclo[4.4.0]deca-4,6-dien-2-yl]-(3R,5R)-sodium heptanoate::CHEMBL690::CS-514::Eptastatin Sodium::PRAVASTATIN SODIUM::Pravachol::Pravastatin::SQ-31000::Sodium; (3R,5R)-3,5-dihydroxy-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-((S)-2-methyl-butyryloxy)-1,2,6,7,8,8a-hexahydro-naphthalen-1-yl]-heptanoate::Sodium; 3,5-dihydroxy-7-[6-hydroxy-2-methyl-8-(2-methyl-butyryloxy)-1,2,6,7,8,8a-hexahydro-naphthalen-1-yl]-heptanoate
SMILES: CC[C@H](C)C(=O)O[C@H]1C[C@@H](O)C=C2C=C[C@H](C)[C@H](CCC(O)C[C@@H](O)CC([O-])=O)[C@@H]12
InChI Key: InChIKey=TUZYXOIXSAXUGO-IRJWOIIVSA-M
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HMG-CoA reductase (Rattus norvegicus (rat)) | BDBM50006412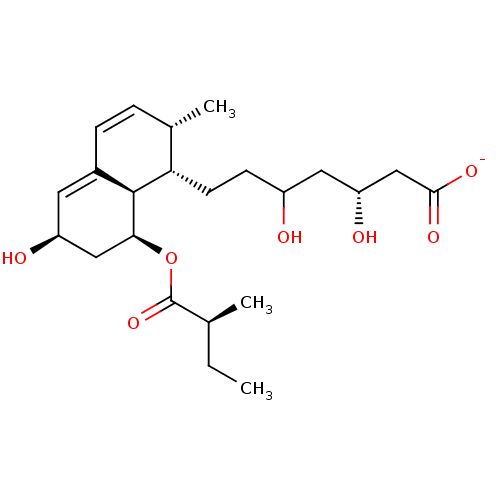 ((3R,5R)-3,5-dihydroxy-7-((1S,2S,6S,8S,8aR)-6-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibition of rat liver microsomal HMG-CoA reductase | J Med Chem 36: 3658-62 (1994) BindingDB Entry DOI: 10.7270/Q2SJ1JP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA) (Homo sapiens (Human)) | BDBM50006412 ((3R,5R)-3,5-dihydroxy-7-((1S,2S,6S,8S,8aR)-6-hydro...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description In vitro HMG-CoA reductase inhibitory activity of the compound to inhibit cellular steroidgenesis in Hep G2 cells (human hepatoma cell line) | Bioorg Med Chem Lett 11: 1285-8 (2001) BindingDB Entry DOI: 10.7270/Q26D5S8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HMG-CoA reductase (Rattus norvegicus (rat)) | BDBM50006412 ((3R,5R)-3,5-dihydroxy-7-((1S,2S,6S,8S,8aR)-6-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description In vitro HMG-CoA reductase inhibitory activity of the compound to inhibit sterol synthesis in cell free system in rat | Bioorg Med Chem Lett 11: 1285-8 (2001) BindingDB Entry DOI: 10.7270/Q26D5S8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||