

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50006842 2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-isopropoxy-phenyl)-3H-quinazolin-4-one::CHEMBL37312
SMILES: CC(C)Oc1cccc(c1)-n1c(CCc2c[nH]c3ccc(Br)cc23)nc2ccccc2c1=O
InChI Key: InChIKey=KUECXUACQOYKNB-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006842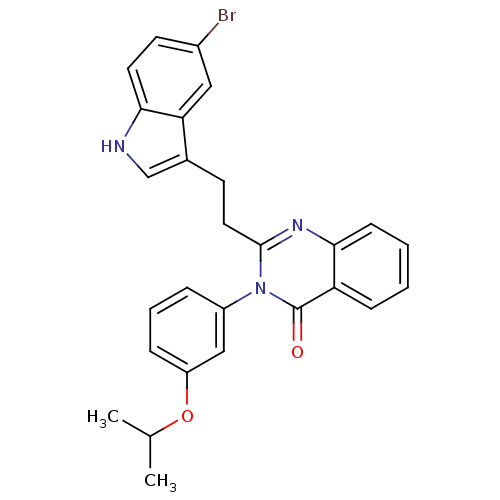 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-isopropox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Evaluated for inhibition of [125I]-CCK-8S binding to cholecystokinin CCK-B receptor from mouse brain membranes at a concentration of 10 microM (in vi... | J Med Chem 35: 2534-42 (1992) BindingDB Entry DOI: 10.7270/Q2JM28MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50006842 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-isopropox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled gastrin binding to gastrin/cholecystokinin type B receptor | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50006842 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-isopropox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled CCK-8 sulfate binding to Cholecystokinin type B receptor in guinea pig brain membranes | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006842 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-isopropox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125 I]CCK-8 binding to Cholecystokinin type B receptor of mouse cerebral cortex | Bioorg Med Chem Lett 7: 805-810 (1997) Article DOI: 10.1016/S0960-894X(97)00108-X BindingDB Entry DOI: 10.7270/Q2XW4JTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50006842 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-isopropox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonistic activity against cholecystokinin type B receptor | J Med Chem 36: 2051-8 (1993) BindingDB Entry DOI: 10.7270/Q2NP2524 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006842 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-isopropox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||