

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50010215 4-Chloro-8-methyl-7-(3-methyl-but-2-enyl)-6,7,8,9-tetrahydro-2H-2,7,9a-triaza-benzo[cd]azulene-1-thione::CHEMBL296092::R(-) 4-Chloro-8-methyl-7-(3-methyl-but-2-enyl)-6,7,8,9-tetrahydro-2H-2,7,9a-triaza-benzo[cd]azulene-1-thione::S(+) 4-Chloro-8-methyl-7-(3-methyl-but-2-enyl)-6,7,8,9-tetrahydro-2H-2,7,9a-triaza-benzo[cd]azulene-1-thione
SMILES: CC1Cn2c3c(CN1CC=C(C)C)cc(Cl)cc3[nH]c2=S
InChI Key: InChIKey=RCSLUNOLLUVOOG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50010215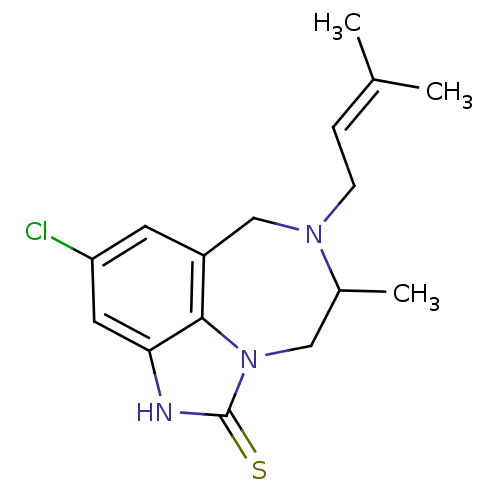 (4-Chloro-8-methyl-7-(3-methyl-but-2-enyl)-6,7,8,9-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to Human immunodeficiency virus 1 reverse transcriptase | Citation and Details Article DOI: 10.1007/s00044-011-9742-x BindingDB Entry DOI: 10.7270/Q2CV4MN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50010215 (4-Chloro-8-methyl-7-(3-methyl-but-2-enyl)-6,7,8,9-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
IBM Thomas J. Watson Research Center Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 replication by interfering with virus reverse transcriptase | J Med Chem 39: 2129-40 (1996) Article DOI: 10.1021/jm950589q BindingDB Entry DOI: 10.7270/Q2697471 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||