

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50010593 1-(2-Chloro-5-isopropylidene-5H-dibenzo[a,d]cyclohepten-10-yl)-4-methyl-piperazine::CHEMBL328246
SMILES: [#6]-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6]-1=[#6]-c2cc(Cl)ccc2\[#6](=[#6](/[#6])-[#6])-c2ccccc-12
InChI Key: InChIKey=QCJIDMPIJUWNKR-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (RAT) | BDBM50010593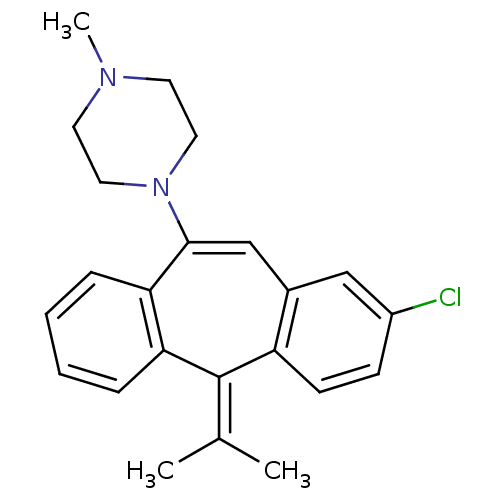 (1-(2-Chloro-5-isopropylidene-5H-dibenzo[a,d]cycloh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity of the compound was evaluated as inhibition constant for dopamine receptor D1 using [3H]-SCH- 23390 as radioligand | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 3a (5-HT3a)/3b (5-HT3b) receptor (Rattus norvegicus-RAT) | BDBM50010593 (1-(2-Chloro-5-isopropylidene-5H-dibenzo[a,d]cycloh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-GR-65,630 binding to NG108-15 cell transfected with cloned rat 5-hydroxytryptamine 3 receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50010593 (1-(2-Chloro-5-isopropylidene-5H-dibenzo[a,d]cycloh...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2A receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin receptor 2a and 2c (5HT2A and 5HT2C) (Rattus norvegicus (Rat)) | BDBM50010593 (1-(2-Chloro-5-isopropylidene-5H-dibenzo[a,d]cycloh...) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity of the compound was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2C recept... | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50010593 (1-(2-Chloro-5-isopropylidene-5H-dibenzo[a,d]cycloh...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-2(long) receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50010593 (1-(2-Chloro-5-isopropylidene-5H-dibenzo[a,d]cycloh...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-4 receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010593 (1-(2-Chloro-5-isopropylidene-5H-dibenzo[a,d]cycloh...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Binding affinity for Dopamine receptor D2 using [3H]-spiperone in rat brain | J Med Chem 33: 809-14 (1990) BindingDB Entry DOI: 10.7270/Q2348JCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50010593 (1-(2-Chloro-5-isopropylidene-5H-dibenzo[a,d]cycloh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 443 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Binding affinity against Dopamine receptor D1 using [3H]-SCN 23390 in rat brain | J Med Chem 33: 809-14 (1990) BindingDB Entry DOI: 10.7270/Q2348JCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (RAT) | BDBM50010593 (1-(2-Chloro-5-isopropylidene-5H-dibenzo[a,d]cycloh...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Binding affinity was determined against Muscarinic acetylcholine receptor using [3H]QNB as radioligand in rat brain. | J Med Chem 33: 809-14 (1990) BindingDB Entry DOI: 10.7270/Q2348JCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||