

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50013635 24-(1H-5-imidazolylmethyl)-13-[1-methyl-(1S)-propyl]-16-(2-naphthylmethyl)perhydrodipyrido[1,2-a:1,2-d]pyrrolo[1,2-j][1,4,7,10,13,16]hexaazacyclooctadecine-6,12,15,18,23,26-hexaone::CHEMBL2372927
SMILES: [H][C@]12CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@]1([H])CCCCN1C(=O)[C@]1([H])CCCCN1C(=O)[C@@]([H])(NC(=O)[C@H](Cc1ccc3ccccc3c1)NC2=O)[C@@H](C)CC
InChI Key: InChIKey=ILLSOIRJMQNTIA-BQMAELMESA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (RAT) | BDBM50013635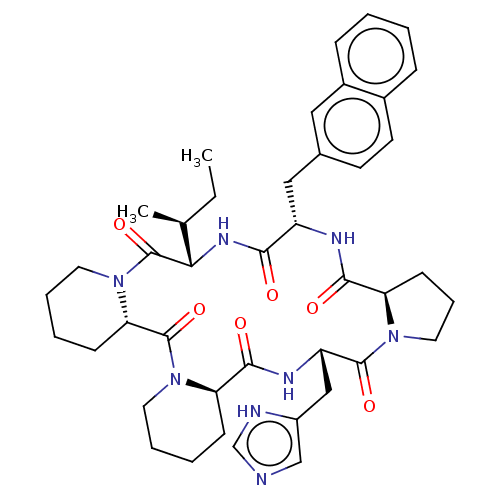 (24-(1H-5-imidazolylmethyl)-13-[1-methyl-(1S)-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 Receptor (Rattus norvegicus (Rat)) | BDBM50013635 (24-(1H-5-imidazolylmethyl)-13-[1-methyl-(1S)-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-arginine vasopressin binding to rat kidney medulla Vasopressin V2 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1 receptor (RAT) | BDBM50013635 (24-(1H-5-imidazolylmethyl)-13-[1-methyl-(1S)-propy...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]arginine vasopressin binding to rat liver Vasopressin V1 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||