

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50016849 CHEMBL3276410
SMILES: [Br-].[Br-].C(CCC[n+]1cc2ccccc2c2ccccc12)CC[n+]1cc2ccccc2c2ccccc12
InChI Key: InChIKey=GENORUDGXFJMHT-UHFFFAOYSA-L
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016849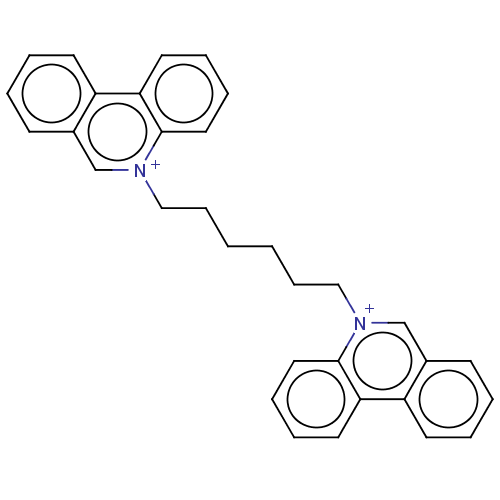 (CHEMBL3276410) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016849 (CHEMBL3276410) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BChE) (Equus caballus (Horse)) | BDBM50016849 (CHEMBL3276410) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BChE) (Equus caballus (Horse)) | BDBM50016849 (CHEMBL3276410) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||