

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50017479 2-(5,9-Diamino-2-methyl-4-oxo-nonanoylamino)-3-phenyl-propionic acid::CHEMBL28708
SMILES: CC(CC(=O)[C@@H](N)CCCCN)C(=O)NC(Cc1ccccc1)C(O)=O
InChI Key: InChIKey=NIMLXPSSVMNDFV-SIWZUTFBSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase B (Rattus norvegicus) | BDBM50017479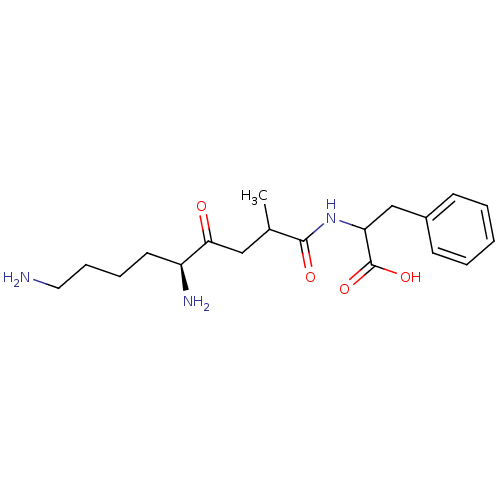 (2-(5,9-Diamino-2-methyl-4-oxo-nonanoylamino)-3-phe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Non-competitive inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver; Ki value reporting the intercept effect(Kii) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50017479 (2-(5,9-Diamino-2-methyl-4-oxo-nonanoylamino)-3-phe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Non-competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the intercept effect(Kii) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50017479 (2-(5,9-Diamino-2-methyl-4-oxo-nonanoylamino)-3-phe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017479 (2-(5,9-Diamino-2-methyl-4-oxo-nonanoylamino)-3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017479 (2-(5,9-Diamino-2-methyl-4-oxo-nonanoylamino)-3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||