

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50026824 CHEMBL3331519::US9669035, B-16
SMILES: CCCOc1cncc(c1)-c1nnc2c(C)nc3ccc(CN4CCOCC4)cc3n12
InChI Key: InChIKey=RXQBGBZOHKWYBQ-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphodiesterase Type 10 (PDE10A) (Rattus norvegicus (rat)) | BDBM50026824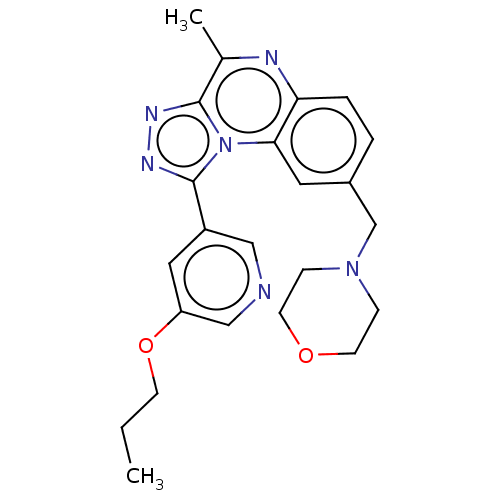 (CHEMBL3331519 | US9669035, B-16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of rat PDE10A expressed in Sf9 cells incubated for 60 mins using [3H]-cAMP substrate by scintillation counting method | ACS Med Chem Lett 5: 1049-53 (2014) Article DOI: 10.1021/ml500262u BindingDB Entry DOI: 10.7270/Q2QN68DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase Type 10 (PDE10A) (Rattus norvegicus (rat)) | BDBM50026824 (CHEMBL3331519 | US9669035, B-16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | 7.8 | 25 |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description Rat recombinant PDE10A (rPDE10A2) was expressed in Sf9 cells using a recombinant rPDE10A baculovirus construct. Cells were harvested after 48 h of in... | US Patent US9669035 (2017) BindingDB Entry DOI: 10.7270/Q2Z60M6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Homo sapiens phosphodiesterase 2A (PDE2A) (Homo sapiens (Human)) | BDBM50026824 (CHEMBL3331519 | US9669035, B-16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.8 | 25 |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description Human recombinant PDE2A (hPDE2A) was expressed in Sf9 cells using a recombinant rPDE10A baculovirus construct. Cells were harvested after 48 h of inf... | US Patent US9669035 (2017) BindingDB Entry DOI: 10.7270/Q2Z60M6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Homo sapiens phosphodiesterase 2A (PDE2A) (Homo sapiens (Human)) | BDBM50026824 (CHEMBL3331519 | US9669035, B-16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV Curated by ChEMBL | Assay Description Inhibition of human PDE2A expressed in Sf9 cells incubated for 40 mins using [3H]-cGMP substrate by scintillation counting method | ACS Med Chem Lett 5: 1049-53 (2014) Article DOI: 10.1021/ml500262u BindingDB Entry DOI: 10.7270/Q2QN68DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||