

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50028333 6-(4-Butyl-phenylamino)-1H-pyrimidine-2,4-dione::CHEMBL54362
SMILES: CCCCc1ccc(Nc2cc(=O)[nH]c(=O)[nH]2)cc1
InChI Key: InChIKey=XIKQUZTWHPFJKG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA topoisomerase III (Bacillus subtilis) | BDBM50028333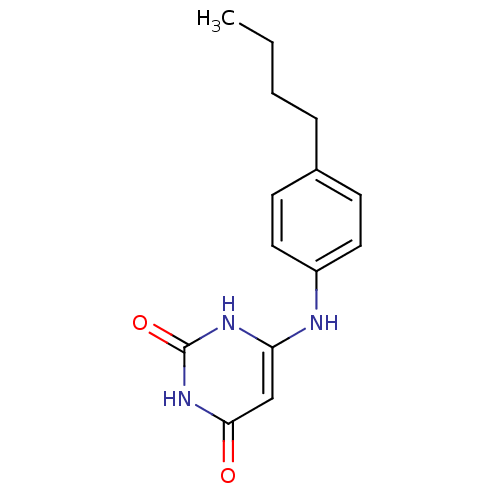 (6-(4-Butyl-phenylamino)-1H-pyrimidine-2,4-dione | ...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.19E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant against Bacillus subtilis azp-12 DNA topoisomerase III (mutant enzyme). | J Med Chem 27: 181-5 (1984) BindingDB Entry DOI: 10.7270/Q2Q241FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase IIIC (Bacillus subtilis) | BDBM50028333 (6-(4-Butyl-phenylamino)-1H-pyrimidine-2,4-dione | ...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.19E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Bacillus subtilis DNA Polymerase III/azp-12(mutant). | J Med Chem 23: 34-8 (1980) BindingDB Entry DOI: 10.7270/Q2125RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase III (Bacillus subtilis) | BDBM50028333 (6-(4-Butyl-phenylamino)-1H-pyrimidine-2,4-dione | ...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.95E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant against Bacillus subtilis DNA topoisomerase III (wild type). | J Med Chem 27: 181-5 (1984) BindingDB Entry DOI: 10.7270/Q2Q241FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase III (Bacillus subtilis) | BDBM50028333 (6-(4-Butyl-phenylamino)-1H-pyrimidine-2,4-dione | ...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.96E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition against Bacillus subtilis DNA topoisomerase III (wild type) | J Med Chem 23: 34-8 (1980) BindingDB Entry DOI: 10.7270/Q2125RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (UNG) (Homo sapiens (Human)) | BDBM50028333 (6-(4-Butyl-phenylamino)-1H-pyrimidine-2,4-dione | ...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 UDG (Herpes Simplex virus type-I Uracil DNA glycosylase) | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||