

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50030538 CHEMBL3344497::US9566312, Compound 2.5.18
SMILES: [H][C@@]1([C@H](C)CN2CCC(F)(F)C2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C
InChI Key: InChIKey=GXYKQROUIHVBLB-DBMJAXCJSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50030538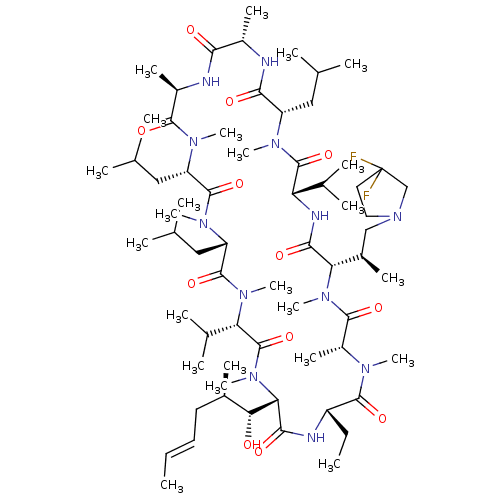 (CHEMBL3344497 | US9566312, Compound 2.5.18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Binding affinity to human cyclophilin A by surface plasmon resonance method | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (OATP1B1) (Homo sapiens (Human)) | BDBM50030538 (CHEMBL3344497 | US9566312, Compound 2.5.18) | UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase F, mitochondrial (Homo sapiens (Human)) | BDBM50030538 (CHEMBL3344497 | US9566312, Compound 2.5.18) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 2 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG US Patent | Assay Description Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... | US Patent US9566312 (2017) BindingDB Entry DOI: 10.7270/Q2K939J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclophilin B (CypB) (Homo sapiens (Human)) | BDBM50030538 (CHEMBL3344497 | US9566312, Compound 2.5.18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG US Patent | Assay Description Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... | US Patent US9566312 (2017) BindingDB Entry DOI: 10.7270/Q2K939J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclophilin A (Homo sapiens (Human)) | BDBM50030538 (CHEMBL3344497 | US9566312, Compound 2.5.18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG US Patent | Assay Description Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... | US Patent US9566312 (2017) BindingDB Entry DOI: 10.7270/Q2K939J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||