

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50031055 3-(3-Methoxy-phenyl)-indeno[1,2-c]pyridazin-5-one::3-(3-methoxyphenyl)-5H-indeno[1,2-c]pyridazin-5-one::CHEMBL125094
SMILES: COc1cccc(c1)-c1cc2C(=O)c3ccccc3-c2nn1
InChI Key: InChIKey=VMMVYTINFCBHDT-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoamine Oxidase Type B (MAO-B) (Rattus norvegicus (rat)) | BDBM50031055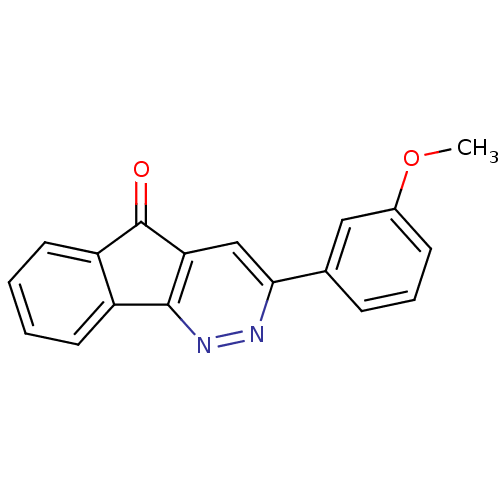 (3-(3-Methoxy-phenyl)-indeno[1,2-c]pyridazin-5-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Neuchâtel Curated by ChEMBL | Assay Description Inhibitory activity against Monoamine oxidase B | J Med Chem 41: 3812-20 (1998) Article DOI: 10.1021/jm981005y BindingDB Entry DOI: 10.7270/Q2F18XVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine oxidase (Rattus norvegicus (rat)) | BDBM50031055 (3-(3-Methoxy-phenyl)-indeno[1,2-c]pyridazin-5-one ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lausanne Curated by ChEMBL | Assay Description Ability to inhibit Monoamine oxidase A enzyme | J Med Chem 38: 3874-83 (1995) BindingDB Entry DOI: 10.7270/Q26972M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50031055 (3-(3-Methoxy-phenyl)-indeno[1,2-c]pyridazin-5-one ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42.7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva Curated by ChEMBL | Assay Description Inhibition of human supersomes MAOB | J Med Chem 49: 6264-72 (2006) Article DOI: 10.1021/jm060441e BindingDB Entry DOI: 10.7270/Q2X63P5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine Oxidase Type B (MAO-B) (Rattus norvegicus (rat)) | BDBM50031055 (3-(3-Methoxy-phenyl)-indeno[1,2-c]pyridazin-5-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva Curated by ChEMBL | Assay Description Inhibition of rat brain MAOB | J Med Chem 49: 6264-72 (2006) Article DOI: 10.1021/jm060441e BindingDB Entry DOI: 10.7270/Q2X63P5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine Oxidase Type B (MAO-B) (Rattus norvegicus (rat)) | BDBM50031055 (3-(3-Methoxy-phenyl)-indeno[1,2-c]pyridazin-5-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lausanne Curated by ChEMBL | Assay Description Ability to inhibit Monoamine oxidase B enzyme | J Med Chem 38: 3874-83 (1995) BindingDB Entry DOI: 10.7270/Q26972M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||