

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50034154 CHEMBL17506::N-Hexyl-N-((4aR,6aS,7S,11aR)-1,4a,6a-trimethyl-2-oxo-hexadecahydro-indeno[5,4-f]quinolin-7-yl)-formamide
SMILES: CCCCCCN(C=O)[C@H]1CCC2C3CC[C@H]4N(C)C(=O)CC[C@]4(C)C3CC[C@]12C
InChI Key: InChIKey=GMEAODWEIDSCAS-XVJZUSICSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5α-Reductase 1 (5α-R1) (Homo sapiens (Human)) | BDBM50034154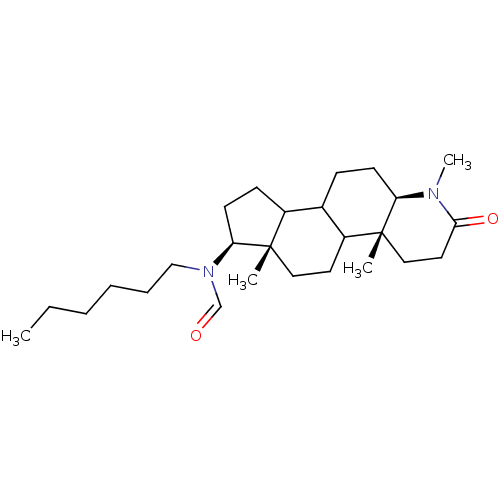 (CHEMBL17506 | N-Hexyl-N-((4aR,6aS,7S,11aR)-1,4a,6a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type I in Du-145 cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Mus musculus) | BDBM50034154 (CHEMBL17506 | N-Hexyl-N-((4aR,6aS,7S,11aR)-1,4a,6a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of DHT (dihydrotestosterone) on proliferation of androgen-sensitive cancer Schionogi (SC-3) cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 5-alpha-reductase (Homo sapiens (Human)) | BDBM50034154 (CHEMBL17506 | N-Hexyl-N-((4aR,6aS,7S,11aR)-1,4a,6a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human steroid 5-alpha-reductase type 2 in SW-13-transfected cells | J Med Chem 38: 1158-73 (1995) BindingDB Entry DOI: 10.7270/Q2NG4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||