

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)(O)c1cn(c(n1)C(C)(C)c1ccccc1Cl)-c1ccc(cc1)-c1cccc(c1)S(C)(=O)=O
InChI Key: InChIKey=JLPURTXCSILYLW-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50034775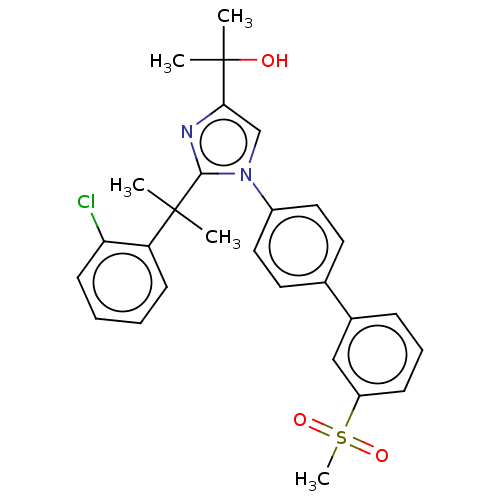 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50034775 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034775 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human LXR-beta expressed in African green monkey CV1 cells measured after 18 to 20 hrs by luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034775 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human LXR-alpha expressed in African green monkey CV1 cells measured after 18 to 20 hrs by luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034775 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a |
The Rockefeller University US Patent | Assay Description As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass... | US Patent US10945978 (2021) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034775 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human HeLa cells assessed as induction of ABCA1 by beta-galactosidase/luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034775 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human whole blood assessed as ABCG1 gene induction by measuring ABCA1 mRNA level after 4 hrs by SYBR-Green dye-based ... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034775 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a |
The Rockefeller University US Patent | Assay Description As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass... | US Patent US10543183 (2020) BindingDB Entry DOI: 10.7270/Q2JW8H8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034775 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a |
The Rockefeller University US Patent | Assay Description As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass... | US Patent US10543183 (2020) BindingDB Entry DOI: 10.7270/Q2JW8H8C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034775 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a |
The Rockefeller University US Patent | Assay Description As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass... | US Patent US10945978 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50034775 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034775 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034775 (CHEMBL3360975 | US10543183, Compound 38 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human whole blood assessed as ABCA1 gene induction by measuring ABCA1 mRNA level after 4 hrs by SYBR-Green dye-based ... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||