

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50037076 CHEMBL3355737
SMILES: O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2cc([nH]n2)C2CCCC2)n1)S(=O)(=O)c1ccccc1
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50037076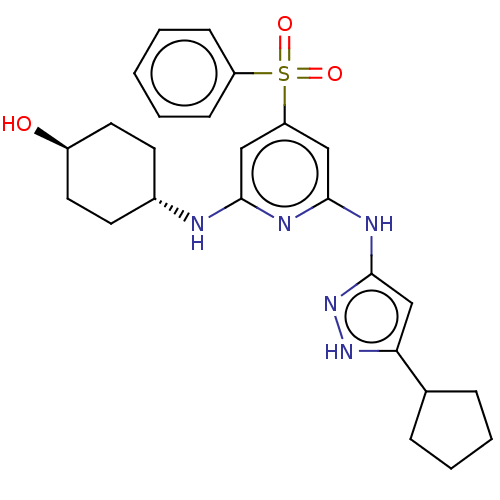 (CHEMBL3355737) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 24: 5818-23 (2014) Article DOI: 10.1016/j.bmcl.2014.10.020 BindingDB Entry DOI: 10.7270/Q2NK3GNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase C-gamma-1 (Homo sapiens (Human)) | BDBM50037076 (CHEMBL3355737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd Curated by ChEMBL | Assay Description Inhibition of PLC gamma-1 phosphorylation in human Jurkat T cells after 30 mins | Bioorg Med Chem Lett 24: 5818-23 (2014) Article DOI: 10.1016/j.bmcl.2014.10.020 BindingDB Entry DOI: 10.7270/Q2NK3GNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||