

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50041963 CHEMBL24861::{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3,4-dihydro-quinazolin-6-yl}-methyl-carbamic acid isobutyl ester::{2-Butyl-4-oxo-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3,4-dihydro-quinazolin-6-yl}-methyl-carbamic acid isobutyl ester
SMILES: CCCCc1nc2ccc(cc2c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)N(C)C(=O)OCC(C)C
InChI Key: InChIKey=UCZOYWFJUMVFHA-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin II type 1a (AT-1a) receptor (RABBIT) | BDBM50041963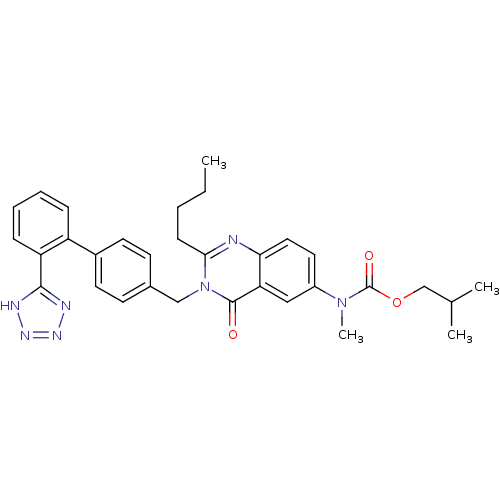 (CHEMBL24861 | {2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [125I]-Sar1-Ile8-AII from angiotensin II receptor, type 1 in rabbit aorta in presence of 0.2% bovine serum albumin (BSA) was dete... | Bioorg Med Chem Lett 3: 1299-1304 (1993) Article DOI: 10.1016/S0960-894X(00)80335-2 BindingDB Entry DOI: 10.7270/Q29P31KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II type 1a (AT-1a) receptor (RABBIT) | BDBM50041963 (CHEMBL24861 | {2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II type 1a (AT-1a) receptor (RABBIT) | BDBM50041963 (CHEMBL24861 | {2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1-Ile8-AII (without BSA) from type 1 Angiotensin II receptor of rabbit aorta | Bioorg Med Chem Lett 3: 1299-1304 (1993) Article DOI: 10.1016/S0960-894X(00)80335-2 BindingDB Entry DOI: 10.7270/Q29P31KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II AT2 (RAT) | BDBM50041963 (CHEMBL24861 | {2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Angiotensin II receptor type 2 from rat mid brain using [125I]-Sar1-Ile8-Ang II without BSA | J Med Chem 36: 3207-10 (1993) BindingDB Entry DOI: 10.7270/Q2QR4W6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II AT2 (RAT) | BDBM50041963 (CHEMBL24861 | {2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity at Angiotensin II type 2 receptor. | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II type 1a (AT-1a) receptor (RABBIT) | BDBM50041963 (CHEMBL24861 | {2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against AT-1 receptor from rabbit aorta using [125I]-Sar1-Ile8-Ang II without BSA | J Med Chem 36: 3207-10 (1993) BindingDB Entry DOI: 10.7270/Q2QR4W6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||