

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50046762 7,8-Dihydroxy-isoquinoline-3-carboxylic acid amide::CHEMBL48384
SMILES: NC(=O)c1cc2ccc(O)c(O)c2cn1
InChI Key: InChIKey=FIBTUUYKTWYAAG-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50046762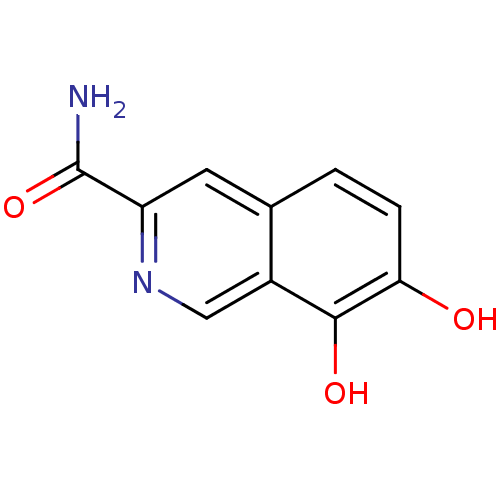 (7,8-Dihydroxy-isoquinoline-3-carboxylic acid amide...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Ability to inhibit autophosphorylation of immunopurified p56Ick | J Med Chem 36: 425-32 (1993) BindingDB Entry DOI: 10.7270/Q2CV4GTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50046762 (7,8-Dihydroxy-isoquinoline-3-carboxylic acid amide...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of p56 lck tyrosine kinase | Bioorg Med Chem Lett 2: 1771-1774 (1992) Article DOI: 10.1016/S0960-894X(00)80473-4 BindingDB Entry DOI: 10.7270/Q2SF2W33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50046762 (7,8-Dihydroxy-isoquinoline-3-carboxylic acid amide...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of epidermal growth factor receptor (EGFR) | Bioorg Med Chem Lett 2: 1771-1774 (1992) Article DOI: 10.1016/S0960-894X(00)80473-4 BindingDB Entry DOI: 10.7270/Q2SF2W33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||