 Found 7 hits for monomerid = 50049823
Found 7 hits for monomerid = 50049823 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heat shock cognate 71 kDa protein
(Homo sapiens (Human)) | BDBM50049823
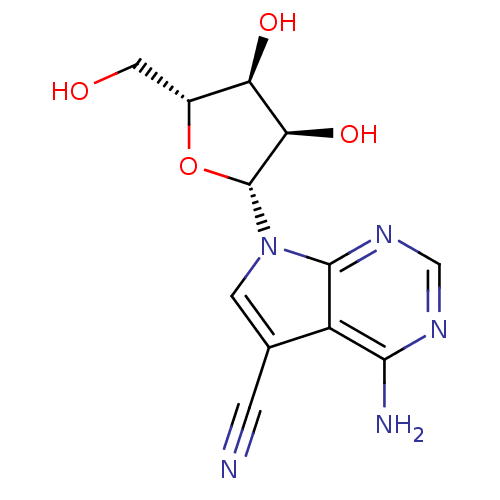
(4-Amino-7-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydroxyme...)Show SMILES Nc1ncnc2n(cc(C#N)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H13N5O4/c13-1-5-2-17(11-7(5)10(14)15-4-16-11)12-9(20)8(19)6(3-18)21-12/h2,4,6,8-9,12,18-20H,3H2,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to human truncated HSC70 NBD (1 to 381 residues) by SPR analysis |
J Med Chem 59: 4625-36 (2016)
Article DOI: 10.1021/acs.jmedchem.5b02001
BindingDB Entry DOI: 10.7270/Q2Z03B3X |
More data for this
Ligand-Target Pair | |
Heat shock cognate 71 kDa protein
(Homo sapiens (Human)) | BDBM50049823

(4-Amino-7-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydroxyme...)Show SMILES Nc1ncnc2n(cc(C#N)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H13N5O4/c13-1-5-2-17(11-7(5)10(14)15-4-16-11)12-9(20)8(19)6(3-18)21-12/h2,4,6,8-9,12,18-20H,3H2,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.12E+4 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to human truncated HSC70 NBD (1 to 381 residues) by SPR analysis |
J Med Chem 59: 4625-36 (2016)
Article DOI: 10.1021/acs.jmedchem.5b02001
BindingDB Entry DOI: 10.7270/Q2Z03B3X |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50049823

(4-Amino-7-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydroxyme...)Show SMILES Nc1ncnc2n(cc(C#N)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H13N5O4/c13-1-5-2-17(11-7(5)10(14)15-4-16-11)12-9(20)8(19)6(3-18)21-12/h2,4,6,8-9,12,18-20H,3H2,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human adenosine kinase |
J Med Chem 43: 2883-93 (2000)
BindingDB Entry DOI: 10.7270/Q2XG9QCV |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50049823

(4-Amino-7-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydroxyme...)Show SMILES Nc1ncnc2n(cc(C#N)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H13N5O4/c13-1-5-2-17(11-7(5)10(14)15-4-16-11)12-9(20)8(19)6(3-18)21-12/h2,4,6,8-9,12,18-20H,3H2,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (unknown origin) using chicken nucleosome as substrate in presence of [3H]SAM incubated for 1 hr by TopCount method |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041
BindingDB Entry DOI: 10.7270/Q25M697H |
More data for this
Ligand-Target Pair | |
Probable global transcription activator SNF2L2
(Homo sapiens (Human)) | BDBM50049823

(4-Amino-7-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydroxyme...)Show SMILES Nc1ncnc2n(cc(C#N)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H13N5O4/c13-1-5-2-17(11-7(5)10(14)15-4-16-11)12-9(20)8(19)6(3-18)21-12/h2,4,6,8-9,12,18-20H,3H2,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | n/a | 9.12E+4 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q2GF0S4R |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50049823

(4-Amino-7-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydroxyme...)Show SMILES Nc1ncnc2n(cc(C#N)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H13N5O4/c13-1-5-2-17(11-7(5)10(14)15-4-16-11)12-9(20)8(19)6(3-18)21-12/h2,4,6,8-9,12,18-20H,3H2,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 309 | n/a | n/a | n/a | n/a | n/a | n/a |
Vigo University
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of the adenosine kinase (AK) activity. |
Bioorg Med Chem Lett 14: 3077-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.040
BindingDB Entry DOI: 10.7270/Q2CZ38BM |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50049823

(4-Amino-7-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydroxyme...)Show SMILES Nc1ncnc2n(cc(C#N)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H13N5O4/c13-1-5-2-17(11-7(5)10(14)15-4-16-11)12-9(20)8(19)6(3-18)21-12/h2,4,6,8-9,12,18-20H,3H2,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data