

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50050533 6-BENZYL-1-BENZYLOXYMETHYL-5-ISOPROPYL URACIL::6-Benzyl-1-benzyloxymethyl-5-isopropyl-1H-pyrimidine-2,4-dione::6-benzyl-1-(benzyloxymethyl)-5-isopropyluracil::CHEMBL436546::TNK-651
SMILES: CC(C)c1c(Cc2ccccc2)n(COCc2ccccc2)c(=O)[nH]c1=O
InChI Key: InChIKey=KSAAUHMSLCPIEX-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50050533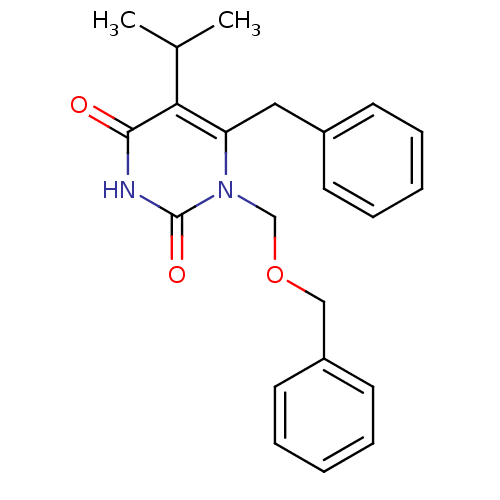 (6-BENZYL-1-BENZYLOXYMETHYL-5-ISOPROPYL URACIL | 6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 reverse transcriptase | J Med Chem 39: 1589-600 (1996) Article DOI: 10.1021/jm960056x BindingDB Entry DOI: 10.7270/Q23X85QC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50050533 (6-BENZYL-1-BENZYLOXYMETHYL-5-ISOPROPYL URACIL | 6-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase expressed in Escherichia coli BL21 (DE3) | J Med Chem 50: 3416-9 (2007) Article DOI: 10.1021/jm070512p BindingDB Entry DOI: 10.7270/Q2765J3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50050533 (6-BENZYL-1-BENZYLOXYMETHYL-5-ISOPROPYL URACIL | 6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(ra)/oligo(dT)15 homopolymer template as substrate after 1 hr | J Med Chem 55: 2242-50 (2012) Article DOI: 10.1021/jm201506e BindingDB Entry DOI: 10.7270/Q22N554J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50050533 (6-BENZYL-1-BENZYLOXYMETHYL-5-ISOPROPYL URACIL | 6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | ACS Med Chem Lett 2: 63-67 (2011) Article DOI: 10.1021/ml1002162 BindingDB Entry DOI: 10.7270/Q2833VW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50050533 (6-BENZYL-1-BENZYLOXYMETHYL-5-ISOPROPYL URACIL | 6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase after 1 hr by colorimetric assay | J Med Chem 56: 3593-608 (2013) Article DOI: 10.1021/jm400102x BindingDB Entry DOI: 10.7270/Q2N019FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50050533 (6-BENZYL-1-BENZYLOXYMETHYL-5-ISOPROPYL URACIL | 6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | J Med Chem 50: 3416-9 (2007) Article DOI: 10.1021/jm070512p BindingDB Entry DOI: 10.7270/Q2765J3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||