

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50051138 2-[(R)-3-(4-Methoxy-benzyl)-2,4,8-trioxo-2,3,4,8-tetrahydro-1H-pyrido[3,4-d]pyrimidin-7-yl]-N-(3,3,3-trifluoro-1-isopropyl-2-oxo-propyl)-acetamide::CHEMBL416159
SMILES: COc1ccc(Cn2c(=O)[nH]c3c(ccn(CC(=O)NC(C(C)C)C(=O)C(F)(F)F)c3=O)c2=O)cc1
InChI Key: InChIKey=PHJQRFRDGYRXSB-UHFFFAOYSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50051138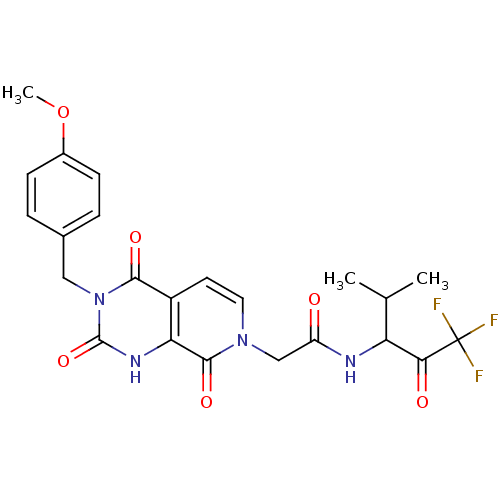 (2-[(R)-3-(4-Methoxy-benzyl)-2,4,8-trioxo-2,3,4,8-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||