

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50051337 2-Phenyl-3,5,7-tripropoxy-chromen-4-one::CHEMBL76273
SMILES: CCCOc1cc(OCCC)c2c(c1)oc(-c1ccccc1)c(OCCC)c2=O
InChI Key: InChIKey=ABZJCSJCNNCZRY-UHFFFAOYSA-N
Data: 5 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50051337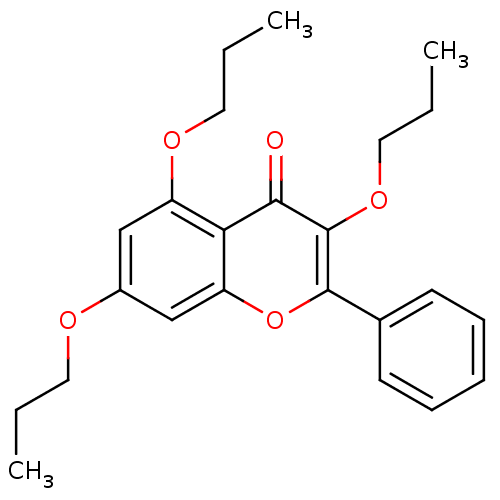 (2-Phenyl-3,5,7-tripropoxy-chromen-4-one | CHEMBL76...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [125I]-AB-MECA binding from adenosine A3 receptor. | J Med Chem 41: 46-52 (1998) Checked by Author Article DOI: 10.1021/jm970446z BindingDB Entry DOI: 10.7270/Q2NC62QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50051337 (2-Phenyl-3,5,7-tripropoxy-chromen-4-one | CHEMBL76...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 317 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity against human adenosine A3 receptor in HEK293 cells using [125I]-AB-MECA 21680 radioligand. | J Med Chem 39: 2293-301 (1996) Article DOI: 10.1021/jm950923i BindingDB Entry DOI: 10.7270/Q2D799J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50051337 (2-Phenyl-3,5,7-tripropoxy-chromen-4-one | CHEMBL76...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]N6-phenylisopropyladenosine binding from adenosine A1 receptor. | J Med Chem 41: 46-52 (1998) Checked by Author Article DOI: 10.1021/jm970446z BindingDB Entry DOI: 10.7270/Q2NC62QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50051337 (2-Phenyl-3,5,7-tripropoxy-chromen-4-one | CHEMBL76...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H]-PIA displacement. | J Med Chem 39: 2293-301 (1996) Article DOI: 10.1021/jm950923i BindingDB Entry DOI: 10.7270/Q2D799J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine Receptors A2a (A2a) (Rattus norvegicus (rat)) | BDBM50051337 (2-Phenyl-3,5,7-tripropoxy-chromen-4-one | CHEMBL76...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]-CGS- 21680 binding from adenosine A2A receptor. | J Med Chem 41: 46-52 (1998) Checked by Author Article DOI: 10.1021/jm970446z BindingDB Entry DOI: 10.7270/Q2NC62QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||