

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50054482 (S)-4-[(S)-2-((S)-1-Formyl-4-guanidino-butylcarbamoyl)-pyrrolidin-1-yl]-4-oxo-3-(2-propyl-pentanoylamino)-butyric acid methyl ester::CHEMBL141676
SMILES: CCCC(CCC)C(=O)N[C@@H](CC(=O)OC)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C=O
InChI Key: InChIKey=ICMSWQUELAGTTR-FHWLQOOXSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50054482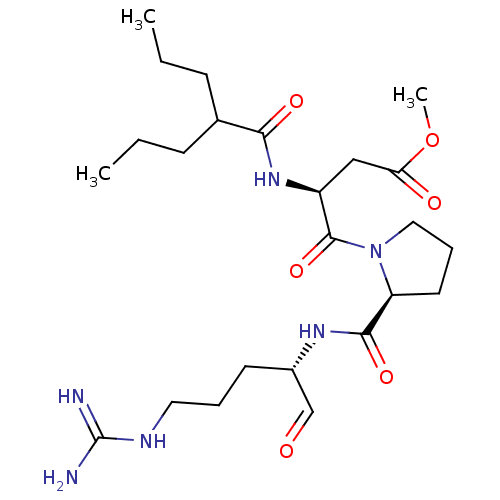 ((S)-4-[(S)-2-((S)-1-Formyl-4-guanidino-butylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 301 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International Inc. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of amidolytic activity for chromogenic substrate Coagulation factor X | J Med Chem 39: 4527-30 (1996) Article DOI: 10.1021/jm960607j BindingDB Entry DOI: 10.7270/Q2CR5SFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin II (Bos taurus) | BDBM50054482 ((S)-4-[(S)-2-((S)-1-Formyl-4-guanidino-butylcarbam...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International Inc. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of amidolytic activity for chromogenic substrate trypsin | J Med Chem 39: 4527-30 (1996) Article DOI: 10.1021/jm960607j BindingDB Entry DOI: 10.7270/Q2CR5SFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054482 ((S)-4-[(S)-2-((S)-1-Formyl-4-guanidino-butylcarbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International Inc. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of amidolytic activity for chromogenic substrate thrombin F11a. | J Med Chem 39: 4527-30 (1996) Article DOI: 10.1021/jm960607j BindingDB Entry DOI: 10.7270/Q2CR5SFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||