

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50056097 1-[(R)-1-(3,3-Dimethyl-2-oxo-butyl)-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl]-3-m-tolyl-urea::CHEMBL115772
SMILES: Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)C(C)(C)C)C2=O)c1
InChI Key: InChIKey=WQULYWDOIINWGJ-SANMLTNESA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCKBR (RAT) | BDBM50056097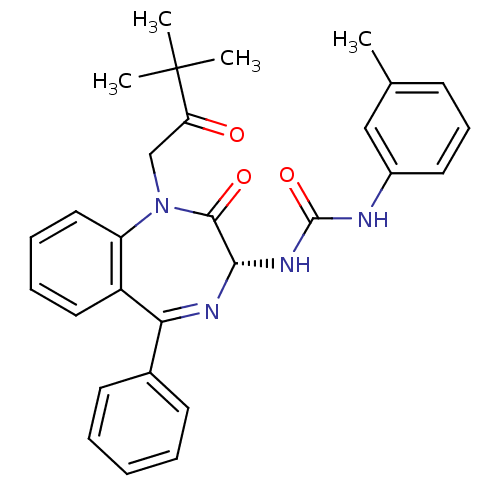 (1-[(R)-1-(3,3-Dimethyl-2-oxo-butyl)-2-oxo-5-phenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Ferring Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brain | J Med Chem 40: 331-41 (1997) Article DOI: 10.1021/jm960669+ BindingDB Entry DOI: 10.7270/Q2VM4BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (RAT) | BDBM50056097 (1-[(R)-1-(3,3-Dimethyl-2-oxo-butyl)-2-oxo-5-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Ferring Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against radioligand [3 H]L-364,718 binding to gastrin/Cholecystokinin type A receptor from rat pancreas | J Med Chem 40: 331-41 (1997) Article DOI: 10.1021/jm960669+ BindingDB Entry DOI: 10.7270/Q2VM4BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (RAT) | BDBM50056097 (1-[(R)-1-(3,3-Dimethyl-2-oxo-butyl)-2-oxo-5-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor from rat pancreas using [I125]-L-364,718 as the radioligand | Bioorg Med Chem Lett 6: 55-58 (1996) Article DOI: 10.1016/0960-894X(95)00557-A BindingDB Entry DOI: 10.7270/Q2CJ8DGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM50056097 (1-[(R)-1-(3,3-Dimethyl-2-oxo-butyl)-2-oxo-5-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]-L-364,718 from Cholecystokinin type A receptor from rat pancreas at dose of 0.03 umol/kg | Bioorg Med Chem Lett 6: 51-54 (1996) Article DOI: 10.1016/0960-894X(95)00556-9 BindingDB Entry DOI: 10.7270/Q2H99553 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CCKBR (RAT) | BDBM50056097 (1-[(R)-1-(3,3-Dimethyl-2-oxo-butyl)-2-oxo-5-phenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards gastrin/Cholecystokinin type B receptor from rat brain using [125I]-CCK-8 as the radioligand | Bioorg Med Chem Lett 6: 55-58 (1996) Article DOI: 10.1016/0960-894X(95)00557-A BindingDB Entry DOI: 10.7270/Q2CJ8DGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CCKBR (RAT) | BDBM50056097 (1-[(R)-1-(3,3-Dimethyl-2-oxo-butyl)-2-oxo-5-phenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [125I]-CCK-8 from gastrin/Cholecystokinin type B receptor from rat brain | Bioorg Med Chem Lett 6: 51-54 (1996) Article DOI: 10.1016/0960-894X(95)00556-9 BindingDB Entry DOI: 10.7270/Q2H99553 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||