

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50057477 4-(3-{3-[(4-Isobutyl-benzyl)-(4-isobutyl-phenyl)-amino]-benzoyl}-indol-1-yl)-butyric acid::4-[3-(3-{[Bis-(4-isobutyl-phenyl)-methyl]-amino}-benzoyl)-indol-1-yl]-butyric acid::CHEMBL25083::FK-143
SMILES: CC(C)Cc1ccc(cc1)C(Nc1cccc(c1)C(=O)c1cn(CCCC(O)=O)c2ccccc12)c1ccc(CC(C)C)cc1
InChI Key: InChIKey=LACIBZRFAYFTOV-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid 5-alpha-reductase (Homo sapiens (Human)) | BDBM50057477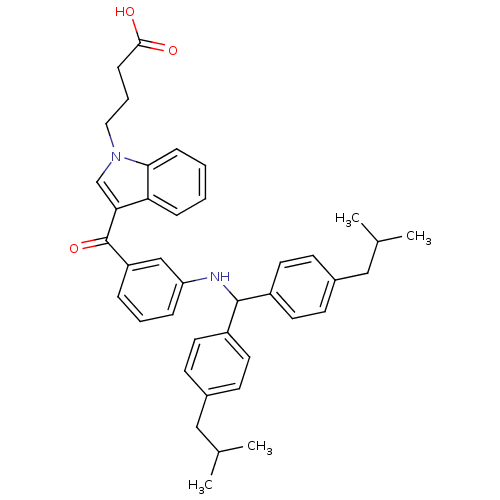 (4-(3-{3-[(4-Isobutyl-benzyl)-(4-isobutyl-phenyl)-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Steroid 5-alpha-reductase type 2 in COS-1 cells was determined | Bioorg Med Chem Lett 8: 561-6 (1999) BindingDB Entry DOI: 10.7270/Q2571B5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5α-Reductase 1 (5α-R1) (Homo sapiens (Human)) | BDBM50057477 (4-(3-{3-[(4-Isobutyl-benzyl)-(4-isobutyl-phenyl)-a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Steroid 5-alpha-reductase type 1 in COS-1 cells was determined | Bioorg Med Chem Lett 8: 561-6 (1999) BindingDB Entry DOI: 10.7270/Q2571B5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 5-alpha-reductase (Rattus norvegicus) | BDBM50057477 (4-(3-{3-[(4-Isobutyl-benzyl)-(4-isobutyl-phenyl)-a...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase from Dawley rat prostate | Bioorg Med Chem Lett 9: 1553-8 (1999) BindingDB Entry DOI: 10.7270/Q2DB841X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5α-Reductase 1 (5α-R1) (Homo sapiens (Human)) | BDBM50057477 (4-(3-{3-[(4-Isobutyl-benzyl)-(4-isobutyl-phenyl)-a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Inhibitory concentration was tested on human 5-alpha reductase 1 isozyme | J Med Chem 40: 1293-315 (1997) Article DOI: 10.1021/jm960697s BindingDB Entry DOI: 10.7270/Q2W096MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 5-alpha-reductase (Rattus norvegicus) | BDBM50057477 (4-(3-{3-[(4-Isobutyl-benzyl)-(4-isobutyl-phenyl)-a...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat testosterone 5 alpha reductase was determined | Bioorg Med Chem Lett 8: 561-6 (1999) BindingDB Entry DOI: 10.7270/Q2571B5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 5-alpha-reductase (Homo sapiens (Human)) | BDBM50057477 (4-(3-{3-[(4-Isobutyl-benzyl)-(4-isobutyl-phenyl)-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Inhibition of human 5-alpha reductase 2 isozyme. | J Med Chem 40: 1293-315 (1997) Article DOI: 10.1021/jm960697s BindingDB Entry DOI: 10.7270/Q2W096MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||