

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50059643 CHEMBL297096::D-Phe-D-ArgNo2-Ome::D-Phenylalanine,methoxy-D-Nitroarginine,with 2M of TFA::methyl 2-[1-amino-2-phenyl-(1R)-ethylcarboxamido]-5-imino(nitroamino)methylamino-(2R)-pentanoate
SMILES: COC(=O)[C@@H](CCCNC(=N)N[N+]([O-])=O)NC(=O)[C@H](N)Cc1ccccc1
InChI Key: InChIKey=DRSRVBDYFUDJCK-CHWSQXEVSA-N
Data: 6 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50059643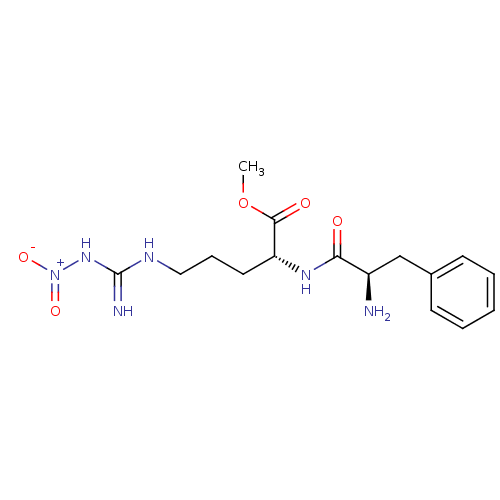 (CHEMBL297096 | D-Phe-D-ArgNo2-Ome | D-Phenylalanin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Neuronal Nitric Oxide Synthase (nNOS) | J Med Chem 46: 1661-9 (2003) Article DOI: 10.1021/jm0202932 BindingDB Entry DOI: 10.7270/Q23J3DQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Nitric Oxide Synthase, brain (Homo sapiens (Human)-Rattus norvegicus (rat)) | BDBM50059643 (CHEMBL297096 | D-Phe-D-ArgNo2-Ome | D-Phenylalanin...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against neuronal nitric oxide synthase | J Med Chem 46: 5700-11 (2003) Checked by Author Article DOI: 10.1021/jm030301u BindingDB Entry DOI: 10.7270/Q26D5TQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric Oxide Synthase, endothelial (Bos taurus (bovine)) | BDBM50059643 (CHEMBL297096 | D-Phe-D-ArgNo2-Ome | D-Phenylalanin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against endothelial nitric oxide synthase (eNOS) | J Med Chem 46: 1661-9 (2003) Article DOI: 10.1021/jm0202932 BindingDB Entry DOI: 10.7270/Q23J3DQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric Oxide Synthase, endothelial (Homo sapiens (Human)-Bos taurus (bovine)) | BDBM50059643 (CHEMBL297096 | D-Phe-D-ArgNo2-Ome | D-Phenylalanin...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against endothelial nitric oxide synthase (eNOS) | J Med Chem 46: 5700-11 (2003) Checked by Author Article DOI: 10.1021/jm030301u BindingDB Entry DOI: 10.7270/Q26D5TQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric Oxide Synthase, inducible (Mus musculus (mouse)-Homo sapiens (Human)) | BDBM50059643 (CHEMBL297096 | D-Phe-D-ArgNo2-Ome | D-Phenylalanin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Inducible nitric oxide synthase | J Med Chem 46: 5700-11 (2003) Checked by Author Article DOI: 10.1021/jm030301u BindingDB Entry DOI: 10.7270/Q26D5TQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric Oxide Synthase, inducible (Mus musculus (mouse)) | BDBM50059643 (CHEMBL297096 | D-Phe-D-ArgNo2-Ome | D-Phenylalanin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Inducible nitric oxide synthase (iNOS) | J Med Chem 46: 1661-9 (2003) Article DOI: 10.1021/jm0202932 BindingDB Entry DOI: 10.7270/Q23J3DQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||