

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50060464 (+) 7-[3-(Biphenyl-4-sulfonylamino)-bicyclo[2.2.1]hept-2-yl]-hept-5-enoic acid::(Z)-7-[(1S,2R,3R,4R)-3-(Biphenyl-4-sulfonylamino)-bicyclo[2.2.1]hept-2-yl]-hept-5-enoic acid::CHEMBL79511
SMILES: OC(=O)CCC\C=C/C[C@@H]1[C@H]2CC[C@H](C2)[C@H]1NS(=O)(=O)c1ccc(cc1)-c1ccccc1
InChI Key: InChIKey=NKFFKDVJGSLYGD-QIXBOIAMSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostanoid DP receptor (Homo sapiens (Human)) | BDBM50060464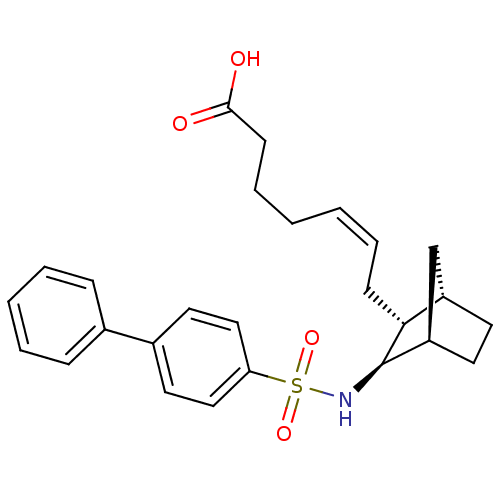 ((+) 7-[3-(Biphenyl-4-sulfonylamino)-bicyclo[2.2.1]...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGD-2 specific binding to Prostaglandin D2 receptor fromhuman platelet membranes | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid IP receptor (Homo sapiens (Human)) | BDBM50060464 ((+) 7-[3-(Biphenyl-4-sulfonylamino)-bicyclo[2.2.1]...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of cAMP formation by carbacyclin in Prostaglandin I2 receptor (IP) assay | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid DP receptor (Homo sapiens (Human)) | BDBM50060464 ((+) 7-[3-(Biphenyl-4-sulfonylamino)-bicyclo[2.2.1]...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of cAMP formation evoked by prostaglandin D2 receptor in human platelets | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid TP receptor (Homo sapiens (Human)) | BDBM50060464 ((+) 7-[3-(Biphenyl-4-sulfonylamino)-bicyclo[2.2.1]...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]- (+)-S-145 specific binding to human platelet membranes in TXA2 receptor (TP) assay | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid DP receptor (Homo sapiens (Human)) | BDBM50060464 ((+) 7-[3-(Biphenyl-4-sulfonylamino)-bicyclo[2.2.1]...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- PGD-2 radioligand binding to prostaglandin D2 receptor on human platelet membrane | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid DP receptor (Homo sapiens (Human)) | BDBM50060464 ((+) 7-[3-(Biphenyl-4-sulfonylamino)-bicyclo[2.2.1]...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of cAMP formation evoked by the prostaglandin D2 receptor in human platelets | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||