

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50066535 (2E)-3-(4-chlorophenyl)-N-[(1R,13R,17S)-4-(cyclopropylmethyl)-10-hydroxy-14-oxo-12-oxa-4-azapentacyclo[9.6.1.0^{1,13}.0^{5,17}.0^{7,18}]octadeca-7,9,11(18)-trien-17-yl]prop-2-enamide::14Beta-4'-Chlorocinnamoylaminodihydronormorphinone::1N-[4-cyclopropylmethyl-10-hydroxy-14-oxo-(17S)-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7,9,11(18)-trien-17-yl]-3-(4-chlorophenyl)-(E)-2-propenamide(Clocinnamox (C-CAM))::CLOCINNAMOX::Clocinnamox
SMILES: [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]5(CCC2=O)NC(=O)\C=C\c1ccc(Cl)cc1)ccc3O
InChI Key: InChIKey=RAURUSFBVQLAPW-OJBLYONPSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50066535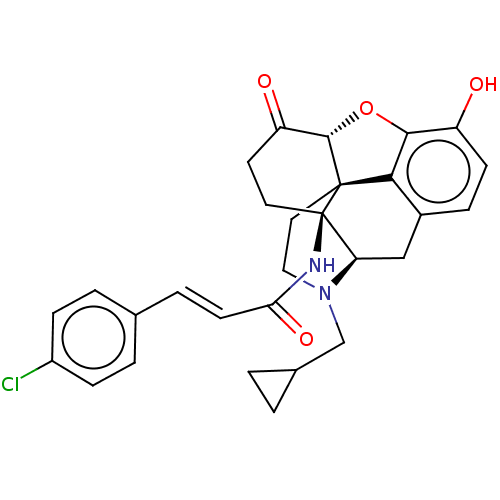 ((2E)-3-(4-chlorophenyl)-N-[(1R,13R,17S)-4-(cyclopr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of radioligand [3H]- DAMGO on Opioid receptor mu 1 in monkey brain membranes | J Med Chem 43: 3348-50 (2000) BindingDB Entry DOI: 10.7270/Q2BZ66R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50066535 ((2E)-3-(4-chlorophenyl)-N-[(1R,13R,17S)-4-(cyclopr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of radioligand [3H]- DPDPE on Opioid receptor delta 1 in monkey brain membranes | J Med Chem 43: 3348-50 (2000) BindingDB Entry DOI: 10.7270/Q2BZ66R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50066535 ((2E)-3-(4-chlorophenyl)-N-[(1R,13R,17S)-4-(cyclopr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of radioligand [3H]- U-69,593 on Opioid receptor kappa 1 in monkey brain membranes | J Med Chem 43: 3348-50 (2000) BindingDB Entry DOI: 10.7270/Q2BZ66R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||