

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: O[B-](=[OH+])c1cccc(c1)[N+]([O-])=O
InChI Key: InChIKey=ZNRGSYUVFVNSAW-UHFFFAOYSA-N
PDB links: 2 PDB IDs match this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase TEM (Escherichia coli) | BDBM50067887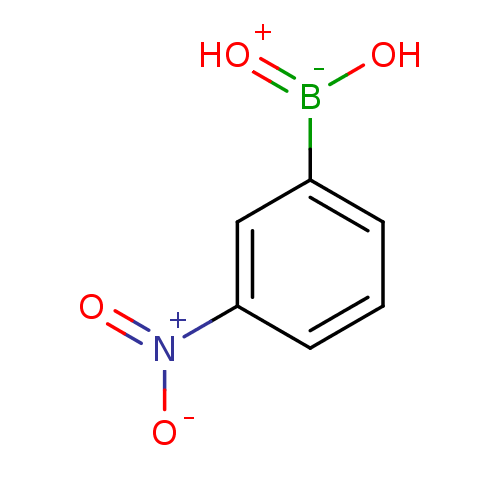 (3-NITROPHENYLBORONIC ACID | 3-Nitro-phenyl-1-dihyd...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article | 1.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant was determined against class A RTEM-1 Beta-lactamase from Escherichia coli | Bioorg Med Chem Lett 4: 1229-1234 (1994) Article DOI: 10.1016/S0960-894X(01)80336-X BindingDB Entry DOI: 10.7270/Q2W9595N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50067887 (3-NITROPHENYLBORONIC ACID | 3-Nitro-phenyl-1-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Medical School Curated by ChEMBL | Assay Description Compound was tested for its specificity against AmpC beta-lactamase | J Med Chem 41: 4577-86 (1998) Article DOI: 10.1021/jm980343w BindingDB Entry DOI: 10.7270/Q22N51FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypsin-2 (Homo sapiens (Human)) | BDBM50067887 (3-NITROPHENYLBORONIC ACID | 3-Nitro-phenyl-1-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Medical School Curated by ChEMBL | Assay Description Compound was tested for its specificity against beta-trypsin | J Med Chem 41: 4577-86 (1998) Article DOI: 10.1021/jm980343w BindingDB Entry DOI: 10.7270/Q22N51FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50067887 (3-NITROPHENYLBORONIC ACID | 3-Nitro-phenyl-1-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Medical School Curated by ChEMBL | Assay Description Compound was tested for its specificity against alpha-chymotrypsin | J Med Chem 41: 4577-86 (1998) Article DOI: 10.1021/jm980343w BindingDB Entry DOI: 10.7270/Q22N51FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Subtilisin BL (Bacillus lentus) | BDBM50067887 (3-NITROPHENYLBORONIC ACID | 3-Nitro-phenyl-1-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of Subtilisin BL M222S-mutated enzyme from B. lentus | Bioorg Med Chem Lett 6: 2501-2506 (1996) Article DOI: 10.1016/0960-894X(96)00466-0 BindingDB Entry DOI: 10.7270/Q2X34XFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50067887 (3-NITROPHENYLBORONIC ACID | 3-Nitro-phenyl-1-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Binding affinity of the compound towards AmpC beta-lactamase binding site from Escherichia coli | J Med Chem 45: 3222-34 (2002) BindingDB Entry DOI: 10.7270/Q2CN737M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Subtilisin Carlsberg (Bacillus licheniformis) | BDBM50067887 (3-NITROPHENYLBORONIC ACID | 3-Nitro-phenyl-1-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of Subtilisin Carlsberg enzyme from B. lentus | Bioorg Med Chem Lett 6: 2501-2506 (1996) Article DOI: 10.1016/0960-894X(96)00466-0 BindingDB Entry DOI: 10.7270/Q2X34XFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Subtilisin BL (Bacillus lentus) | BDBM50067887 (3-NITROPHENYLBORONIC ACID | 3-Nitro-phenyl-1-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of Subtilisin BL wild type enzyme from B. lentus | Bioorg Med Chem Lett 6: 2501-2506 (1996) Article DOI: 10.1016/0960-894X(96)00466-0 BindingDB Entry DOI: 10.7270/Q2X34XFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Subtilisin BL (Bacillus lentus) | BDBM50067887 (3-NITROPHENYLBORONIC ACID | 3-Nitro-phenyl-1-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of Subtilisin BL M222C-mutated enzyme from B. lentus | Bioorg Med Chem Lett 6: 2501-2506 (1996) Article DOI: 10.1016/0960-894X(96)00466-0 BindingDB Entry DOI: 10.7270/Q2X34XFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50067887 (3-NITROPHENYLBORONIC ACID | 3-Nitro-phenyl-1-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Medical School Curated by ChEMBL | Assay Description Inhibitory activity against E. coli AmpC beta-lactamase. | J Med Chem 41: 4577-86 (1998) Article DOI: 10.1021/jm980343w BindingDB Entry DOI: 10.7270/Q22N51FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||