

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50068039 1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)-amino]-propyl}-3-[3-(1H-imidazol-4-yl)-propyl]-thiourea::CHEMBL103769
SMILES: Clc1ccc(CN(CCCNC(=S)NCCCc2cnc[nH]2)c2ccc(Br)cn2)cc1Cl
InChI Key: InChIKey=UREJDUPKGMFJRU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50068039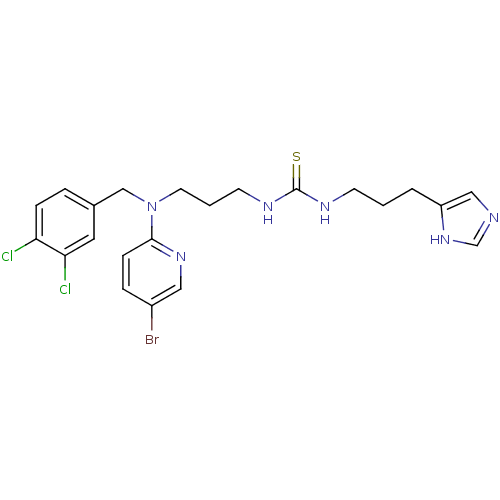 (1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibition of [125 I -Tyr]SRIF-14 binding to membranes isolated from CHO-K1 cells expressing cloned human SRIF receptor (sst-4) subtype | J Med Chem 44: 2990-3000 (2001) BindingDB Entry DOI: 10.7270/Q2DB82JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50068039 (1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeast Louisiana University Curated by ChEMBL | Assay Description In vitro binding affinity at somatostatin receptor 4 in transfected BHK cells using [125 I]Tyr11-SRIF-14 as radioligand | J Med Chem 41: 4693-705 (1998) Article DOI: 10.1021/jm980118e BindingDB Entry DOI: 10.7270/Q2TT4RNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50068039 (1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Binding affinity to human SST4 receptor | Medchemcomm 3: 56-60 (2012) Article DOI: 10.1039/c1md00200g BindingDB Entry DOI: 10.7270/Q2XP77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50068039 (1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 621 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeast Louisiana University Curated by ChEMBL | Assay Description In vitro binding affinity at somatostatin 2 receptor in transfected HEK 293 cell using [125 I]Tyr11-SRIF-14 as radioligand | J Med Chem 41: 4693-705 (1998) Article DOI: 10.1021/jm980118e BindingDB Entry DOI: 10.7270/Q2TT4RNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50068039 (1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase in human spleen homogenate using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to subs... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50068039 (1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to substrate addition measured aft... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||