

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50070051 (4aR,10bR)-4,10b-Dimethyl-8-((E)-styryl)-1,4,4a,5,6,10b-hexahydro-2H-benzo[f]quinolin-3-one::CHEMBL300446
SMILES: CN1[C@@H]2CCc3cc(\C=C\c4ccccc4)ccc3[C@@]2(C)CCC1=O
InChI Key: InChIKey=YLMDSHIOZFJIOC-LZLPKMGPSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5α-Reductase 1 (5α-R1) (Homo sapiens (Human)) | BDBM50070051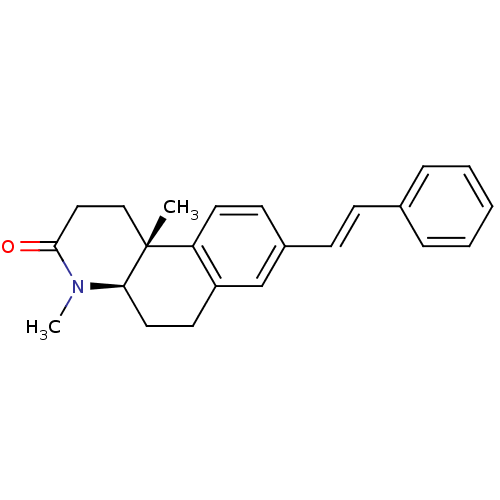 ((4aR,10bR)-4,10b-Dimethyl-8-((E)-styryl)-1,4,4a,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type I enzyme based on the conversion of [3H]-T to [3H]-DHT in nuclear membrane preparations fr... | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5α-Reductase 1 (5α-R1) (Homo sapiens (Human)) | BDBM50070051 ((4aR,10bR)-4,10b-Dimethyl-8-((E)-styryl)-1,4,4a,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description In vitro inhibitory activity against Steroid 5-alpha-reductase type I | Bioorg Med Chem Lett 10: 1909-11 (2001) BindingDB Entry DOI: 10.7270/Q2M044PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 5-alpha-reductase (Homo sapiens (Human)) | BDBM50070051 ((4aR,10bR)-4,10b-Dimethyl-8-((E)-styryl)-1,4,4a,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Steroid 5-alpha-reductase type 2 | Bioorg Med Chem Lett 10: 1909-11 (2001) BindingDB Entry DOI: 10.7270/Q2M044PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 5-alpha-reductase (Homo sapiens (Human)) | BDBM50070051 ((4aR,10bR)-4,10b-Dimethyl-8-((E)-styryl)-1,4,4a,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type 2 as [3H]-T to [3H]-DHT conversion human prostate nuclear membrane | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||