

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(cc3)N3CCNCC3)n[nH]c2c1
InChI Key: InChIKey=FJXHOUJMCHOYIO-QCPDJZBESA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50073555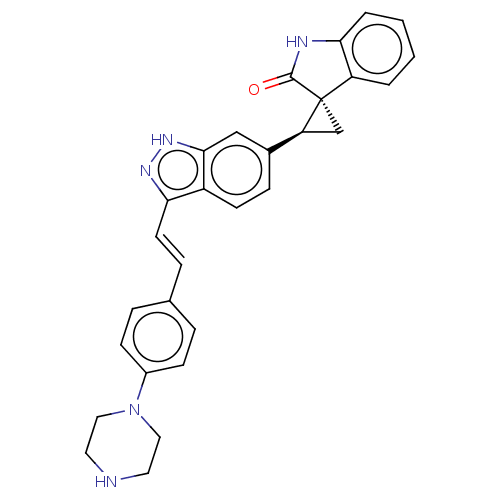 (CHEMBL3408941 | US10358436, Example A92 | US990780...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) by fluorescence assay | J Med Chem 58: 147-69 (2015) BindingDB Entry DOI: 10.7270/Q2M0475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50073555 (CHEMBL3408941 | US10358436, Example A92 | US990780...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) by fluorescence assay | J Med Chem 58: 147-69 (2015) BindingDB Entry DOI: 10.7270/Q2M0475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50073555 (CHEMBL3408941 | US10358436, Example A92 | US990780...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay | J Med Chem 58: 147-69 (2015) BindingDB Entry DOI: 10.7270/Q2M0475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50073555 (CHEMBL3408941 | US10358436, Example A92 | US990780...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PLK4 (unknown origin) by ELISA | J Med Chem 58: 147-69 (2015) BindingDB Entry DOI: 10.7270/Q2M0475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50073555 (CHEMBL3408941 | US10358436, Example A92 | US990780...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description PLK4 activity was measured using an indirect ELISA detection system. Dephosphorylated GST-PLK4 (4 nM) was incubated in the presence of 15 μM ATP... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2KD216W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50073555 (CHEMBL3408941 | US10358436, Example A92 | US990780...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network US Patent | Assay Description Aurora B inhibition was determined using the Z-Lyte assay kit from Invitrogen. The assay was performed using the recommended manufacturer's instr... | US Patent US10358436 (2019) BindingDB Entry DOI: 10.7270/Q2K939WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50073555 (CHEMBL3408941 | US10358436, Example A92 | US990780...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description PLK4 activity was measured using an indirect ELISA detection system. Dephosphorylated GST-PLK4 (4 nM) was incubated in the presence of 15 μM ATP... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2KD216W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50073555 (CHEMBL3408941 | US10358436, Example A92 | US990780...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Aurora B inhibition was determined using the Z-Lyte assay kit from Invitrogen. The assay was performed using the recommended manufacturer's instr... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2KD216W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 [1-391] (Homo sapiens (Human)) | BDBM50073555 (CHEMBL3408941 | US10358436, Example A92 | US990780...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network US Patent | Assay Description Active PLK4 was purified from an E. coli expression system as an amino terminal GST fusion of residues 1-391 of human PLK4. The protein was purified ... | US Patent US10358436 (2019) BindingDB Entry DOI: 10.7270/Q2K939WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50073555 (CHEMBL3408941 | US10358436, Example A92 | US990780...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network US Patent | Assay Description Aurora A inhibition was determined using the Z-Lyte assay kit from Invitrogen. The assay was performed using the recommended manufacturer's instr... | US Patent US10358436 (2019) BindingDB Entry DOI: 10.7270/Q2K939WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50073555 (CHEMBL3408941 | US10358436, Example A92 | US990780...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Aurora B inhibition was determined using the Z-Lyte assay kit from Invitrogen. The assay was performed using the recommended manufacturer's instr... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2KD216W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||