

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50076138 (2S)-methyl 2-(4-((R)-2-amino-3-mercaptopropylamino)-2-(naphthalen-1-yl)benzamido)-4-methylpentanoate::(S)-2-[4-((R)-2-Amino-3-mercapto-propylamino)-2-naphthalen-1-yl-benzoylamino]-4-methyl-pentanoic acid methyl ester::2-[4-((R)-2-Amino-3-mercapto-propylamino)-2-naphthalen-1-yl-benzoylamino]-4-methyl-pentanoic acid (S)-methyl ester::CHEMBL282748
SMILES: COC(=O)[C@H](CC(C)C)NC(=O)c1ccc(NC[C@@H](N)CS)cc1-c1cccc2ccccc12
InChI Key: InChIKey=XVWPFYDMUFBHBF-CLOONOSVSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geranylgeranyl transferase type I beta subunit/Protein Farnesyltransferase (PFT) (Homo sapiens (Human)) | BDBM50076138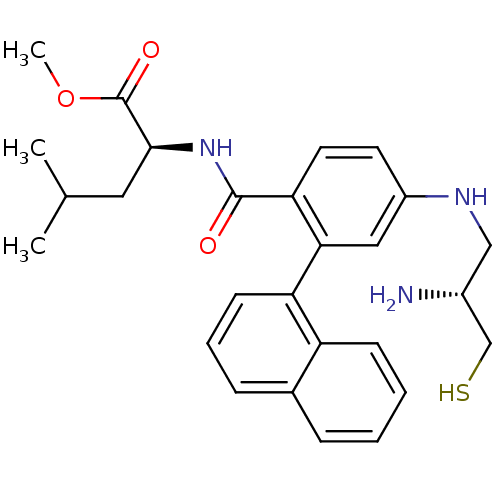 ((2S)-methyl 2-(4-((R)-2-amino-3-mercaptopropylamin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition binding constant Geranylgeranyl transferase type I | J Med Chem 47: 1869-78 (2004) Article DOI: 10.1021/jm0305467 BindingDB Entry DOI: 10.7270/Q2RX9CVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase beta/geranylgeranyltransferase type I alpha subunit (Mus musculus) | BDBM50076138 ((2S)-methyl 2-(4-((R)-2-amino-3-mercaptopropylamin...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description In vivo for inhibition of farnesylation based on the inhibition of H-Ras processing. | J Med Chem 42: 1333-40 (1999) Article DOI: 10.1021/jm9900873 BindingDB Entry DOI: 10.7270/Q28G8JW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||