

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)c1ccc(NC(=O)Oc2ccc3NC4NCC[C@@]4(C)c3c2)cc1
InChI Key: InChIKey=ZIGIADNCAWZUAB-QWAKEFERSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50077257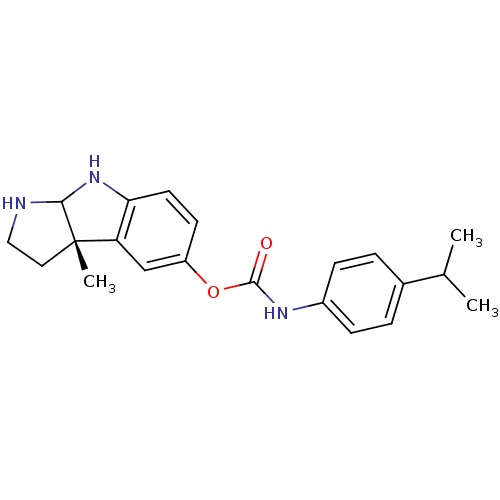 ((4-Isopropyl-phenyl)-carbamic acid (S)-3a-methyl-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Intramural Research Program Curated by ChEMBL | Assay Description Ability to inhibit acetylcholinesterase (AChE), freshly prepared from human erythrocytes | J Med Chem 42: 1855-61 (1999) Article DOI: 10.1021/jm980459s BindingDB Entry DOI: 10.7270/Q22N51GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50077257 ((4-Isopropyl-phenyl)-carbamic acid (S)-3a-methyl-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University W£rzburg Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetyl-(beta-methyl)thiocholine as substrate preincubated for 30 mins followed by substrate addition and m... | J Med Chem 62: 9116-9140 (2019) Article DOI: 10.1021/acs.jmedchem.9b01012 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50077257 ((4-Isopropyl-phenyl)-carbamic acid (S)-3a-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University W£rzburg Curated by ChEMBL | Assay Description Inhibition of human plasma BChE using s-butyrylthiocholine as substrate preincubated for 30 mins followed by substrate addition and measured after 25... | J Med Chem 62: 9116-9140 (2019) Article DOI: 10.1021/acs.jmedchem.9b01012 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50077257 ((4-Isopropyl-phenyl)-carbamic acid (S)-3a-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Intramural Research Program Curated by ChEMBL | Assay Description Ability to inhibit butyrylcholinesterase (BChE), freshly prepared from human plasma | J Med Chem 42: 1855-61 (1999) Article DOI: 10.1021/jm980459s BindingDB Entry DOI: 10.7270/Q22N51GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||