

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50077323 7,4'-Dihydroxyflavone::7-hydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one::CHEMBL294878
SMILES: Oc1ccc(cc1)-c1cc(=O)c2ccc(O)cc2o1
InChI Key: InChIKey=LCAWNFIFMLXZPQ-UHFFFAOYSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-glucuronidase (Rattus norvegicus) | BDBM50077323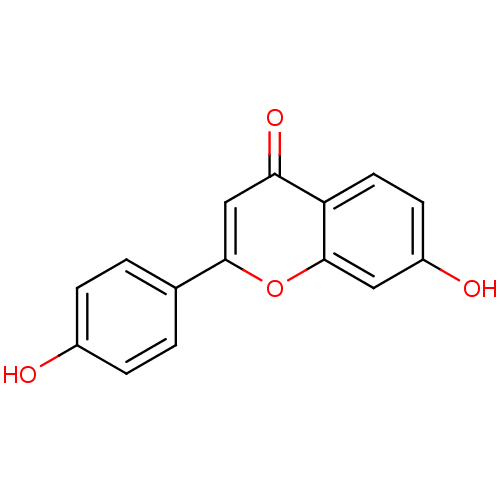 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C (Rattus norvegicus) | BDBM50077323 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldose reductase (ALR2) (Bos taurus (Cattle)) | BDBM50077323 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | PDB GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Modena Curated by ChEMBL | Assay Description Inhibition of ALR2 (aldose reductase) of bovine lens | J Med Chem 42: 1881-93 (1999) Article DOI: 10.1021/jm980441h BindingDB Entry DOI: 10.7270/Q25X29M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50077323 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of p56 lck | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol hexakisphosphate kinase 2 (Homo sapiens) | BDBM50077323 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Environmental Health Sciences Curated by ChEMBL | Assay Description Inhibition of human IP6K2 using insP6 as substrate preincubated for 15 mins followed by substrate and measured after 30 mins by TR-FRET assay | J Med Chem 62: 1443-1454 (2019) Article DOI: 10.1021/acs.jmedchem.8b01593 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase 2 (Homo sapiens (Human)) | BDBM50077323 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Neu2 assessed as MuNANA substrate hydrolysis in presence of 0.1% Triton X-100 by discontinuous fluorimetric assay | Bioorg Med Chem 18: 1633-40 (2010) Article DOI: 10.1016/j.bmc.2009.12.062 BindingDB Entry DOI: 10.7270/Q26110F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM50077323 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 | Bioorg Med Chem Lett 20: 3050-64 (2010) Article DOI: 10.1016/j.bmcl.2010.03.113 BindingDB Entry DOI: 10.7270/Q2CJ8FFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50077323 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibition of cathepsin D. | J Med Chem 60: 6563-6586 (2017) Article DOI: 10.1021/acs.jmedchem.7b00285 BindingDB Entry DOI: 10.7270/Q27D2XD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate multikinase (Homo sapiens) | BDBM50077323 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Environmental Health Sciences Curated by ChEMBL | Assay Description Inhibition of human IPMK using insP3 as substrate preincubated for 15 mins followed by substrate and measured after 30 mins by TR-FRET assay | J Med Chem 62: 1443-1454 (2019) Article DOI: 10.1021/acs.jmedchem.8b01593 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl Cyclase (Homo sapiens (Human)) | BDBM50077323 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenzhen University Curated by ChEMBL | Assay Description Inhibition of human recombinant glutaminyl cyclase expressed in Escherichia coli using H-Gln-Gln-H substrate measured for 15 mins by spectrophotometr... | Bioorg Med Chem 24: 2280-6 (2016) BindingDB Entry DOI: 10.7270/Q2P55QDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||